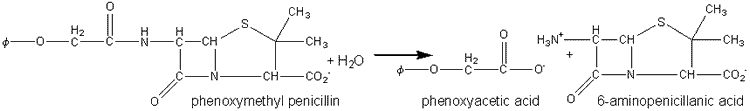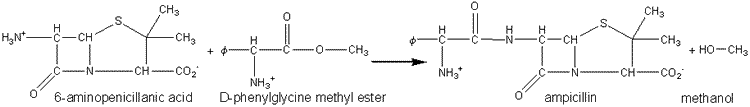|
|
Production of antibiotics
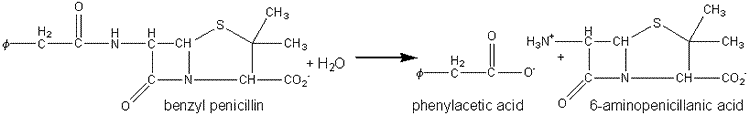
Penicillin amidase may be obtained from E. coli and has been immobilised on a number of supports including cyanogen bromide-activated Sephadex G200. It represents one of the earliest successful processes involving immobilised enzymes and is generally used in batch or semicontinuous STR processes (40,000 Ukg−1penicillin G, 35°C, pH 7.8, 2 h) where it may be reused over 100 times. It has also been used in PBRs, where it has an active life of over 100 days, producing about two tonnes of 6-aminopenicillanic acid kg−1of immobolised enzyme.
The penicillin-G-amidases may be used 'in reverse' to synthesise penicillin and cephalosporin antibiotics by non -equilibrium kinetically controlled reactions (see also Chapter 7). Ampicillin has been produced by the use of penicillin-G-amidase immobilised by adsorption to DEAE -cellulose in a packed bed column:
Many other potential and proven antibiotics have been synthesised in this manner, using a variety of synthetic b-lactams and activated carboxylic acids. This page was established in 2004 and last updated by Martin
Chaplin |