|
|
The mechanism of enzyme catalysis
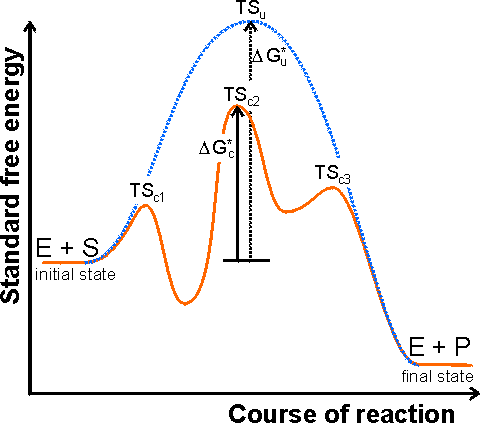
Figure 1.1. A schematic diagram showing the free energy profile of the course of an enzyme catalysed reaction involving the formation of enzyme-substrate (ES) and enzyme-product (EP) complexes, i.e.
The catalysed reaction pathway goes through the transition states TSc1, TSc2 and TSc3, with standard free energy of activation DGc*, whereas the uncatalysed reaction goes through the transition state TSu with standard free energy of activation DGu*. In this example the rate limiting step would be the conversion of ES into EP. Reactions involving several substrates and products, or more intermediates, are even more complicated. The Michaelis-Menten reaction scheme [1.7] would give a similar profile but without the EP-complex free energy trough. The schematic profile for the uncatalysed reaction is shown as the dashed line. It should be noted that the catalytic effect only concerns the lowering of the standard free energy of activation from DGu* to DGc* and has no effect on the overall free energy change (i.e., the difference between the initial and final states) or the related equilibrium constant. There are a number of mechanisms by which this activation energy decrease may be achieved. The most important of these involves the enzyme initially binding the substrate(s), in the correct orientation to react, close to the catalytic groups on the active enzyme complex and any other substrates. In this way the binding energy is used partially in order to reduce the contribution of the considerable activation entropy, due to the loss of the reactants' (and catalytic groups') translational and rotational entropy, towards the total activation energy. Other contributing factors are the introduction of strain into the reactants (allowing more binding energy to be available for the transition state), provision of an alternative reactive pathway and the desolvation of reacting and catalysing ionic groups. The energies available to enzymes for binding their substrates are determined primarily by the complementarity of structures (i.e., a good 3-dimensional fit plus optimal non-covalent ionic and/or hydrogen-bonding forces). The specificity depends upon minimal steric repulsion, the absence of unsolvated or unpaired charges, and the presence of sufficient hydrogen bonds. These binding energies are capable of being quite large. As examples, antibody-antigen dissociation constants are characteristically near 10−8 M (free energy of binding is 46 kJ M−1), ATP binds to myosin with a dissociation constant of 10−13 M (free energy of binding is 75 kJ M−1) and biotin binds to avidin, a protein found in egg white, with a dissociation constant of 10−15 M (free energy of binding is 86 kJ M−1). However, enzymes do not use this potential binding energy simply in order to bind the substrate(s) and form stable long-lasting complexes. If this were to be the case, the formation of the transition state between ES and EP would involve an extremely large free energy change due to the breaking of these strong binding forces, and the rate of formation of products would be very slow. They must use this binding energy for reducing the free energy of the transition state. This is generally achieved by increasing the binding to the transition state rather than the reactants and, in the process, introducing an energetic strain into the system and allowing more favourable interactions between the enzyme's catalytic groups and the reactants.
This page was established in 2004 and last updated by Martin
Chaplin |