YMT2019a
Takuma Yagasaki, Masakazu Matsumoto, and Hideki Tanaka
Liquid-Liquid Separation of Aqueous Solutions: A Molecular Dynamics Study
J. Chem. Phys. 150, 214506 (2019).
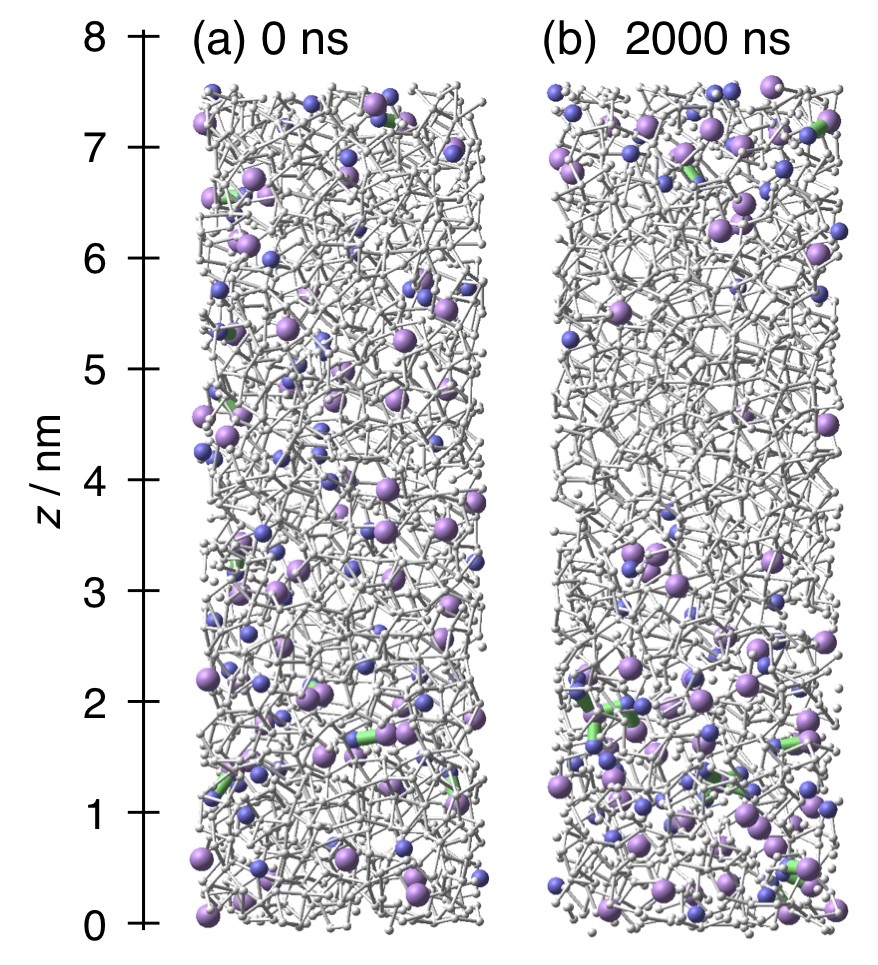
In the liquid-liquid phase transition scenario, supercooled water separates into the high density liquid (HDL) and low density liquid (LDL) phases at temperatures lower than the second critical point. We investigate the effects of hydrophilic and hydrophobic solutes on the liquid-liquid phase transition using molecular dynamics simulations. It is found that a supercooled aqueous NaCl solution separates into solute-rich HDL and solute-poor LDL parts at low pressures. By contrast, a supercooled aqueous Ne solution separates into solute-rich LDL and solute-poor HDL parts at high pressures. Both the solutes increase the high temperature limit of the liquid-liquid separation. The degree of separation is quantified using the local density of solute particles to determine the liquid-liquid coexistence region in the pressure-temperature phase diagram. The effects of NaCl and Ne on the phase diagram of supercooled water are explained in terms of preferential solvation of ions in HDL and that of small hydrophobic particles in LDL, respectively.
液液相転移シナリオでは,過冷却水は第二臨界点より低い温度で高密度液相(HDL)と低密度液相(LDL)に分離する。分子動力学シミュレーションを用いて液‐液相転移に及ぼす親水性及び疎水性溶質の効果を調べた。過冷却NaCl水溶液は低圧で溶質に富むHDL部分と溶質に乏しいLDL部分に分離することが分かった。対照的に,過冷却Ne水溶液は高圧で溶質リッチLDL部分と溶質欠乏HDL部分に分離する。両溶質は液液分離の高温限界を増加させる。溶質粒子の局所密度を用いて分離度を定量化し,圧力‐温度状態図における液‐液共存領域を決定した。過冷却水の相図に及ぼすNaClとNeの影響をHDL中のイオンの優先溶媒和とLDL中の小さな疎水性粒子の優先溶媒和で説明した。(ミライ翻訳による自動翻訳。)
research papers paper2019 polyamorphism solution aqueoussolution