|
|
The stabilisation of enzymes in biphasic aqueous-organic systemsIt should become clear from the later discussion that there may be a substantial advantage to be gained from the use of biphasic systems in many enzyme-catalysed reactions. One major factor must first be addressed; the stability of the enzyme in these systems. A distinction should be drawn between the more water-soluble hydrophilic enzymes and the more hydrophobic enzymes often associated with lipid and membranes (e.g., lipases). The active integrity and stability of hydrophilic enzymes appears to depend on the presence of a thin layer of water, just a few molecules thick, within the microenvironment. This amount of water is miniscule (between 50 and 500 molecules of water for each enzyme molecule) and the enzyme may effectively be operating in an almost anhydrous state. Some hydrophobic lipases retain activity even if fewer molecules of water remain; presumably just sufficient to stabilise the conformation of the active site. The pH of such minute pools of water, containing no free hydrogen ions, is impossible to measure, or control, directly. However, it appears that the enzyme 'remembers' the pH of its last aqueous solution and functions as though at that pH. If the enzyme-bound water is stripped out or diluted by the use of the more water-soluble, or miscible, organic solvents then the enzyme is usually inactivated. However, under conditions where this does not occur, the limited amount of water available, and the associated reduction in the water activity, considerably reduces the rate of thermoinactivation. This has a stabilising effect on most enzymes; porcine pancreatic lipase, for example, has a half-life of greater than 12 hours at 100°C in 0.02% water in tributyrin, whereas this drops to 12 minutes at a 0.8% water content and inactivation is almost instantaneous in 100% water. Additionally, the freezing point of the water is reduced which allows the use of particularly heat-labile enzymes at very low temperatures. The lowering of the water activity tends to produce a more rigid enzyme molecule which may affect both its Km and Vmax. In extreme cases, this may result in a change in the catalytic properties. Porcine pancreatic lipase demonstrates this effect. When used in biphasic systems of low water activity, it no longer catalyzes transesterification reactions involving the bulky tertiary alcohols. The most important factor in the balance between stabilisation and inactivation, due to organic phase, is the solvent polarity. Solvents of lower polarity (i.e., greater hydrophobicity) are less able to disrupt the structure of the necessary tightly bound water molecules. The best measure of polarity is the logarithm of the partition coefficient (LogP) of the organic liquid between n-octanol and water; the higher the LogP, the more non-polar (hydrophobic) is the solvent (Table 7.1).
Table 7.1. LogP values of the more commonly used organic solvents.
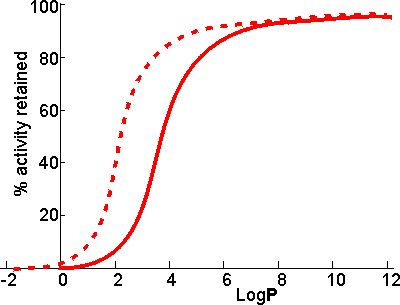
LogP values increase by about 0.52 for every methylene group (-CH2-) added in an homologous series. Thus, the LogP of hexanol is that of butanol (0.8) plus 2 ˣ 0.52 (i.e., approximately 1.8). There appears to be a clear correlation between the activity of biocatalysts in two-phase systems and the LogP (Figure 7.2). The S-shape of this relationship suggests that enzymes are generally inactivated by solvents with logP < 2 but are little affected by solvents with LogP > 4. There is some variation in the effects between different enzymes and different solvents which makes activity prediction, in the LogP range of 2 - 4, rather imprecise. This range includes some of the most utilised organic phases (e.g., chloroform), which may be suitable for some applications but cause harmful inactivation in others. The solubility of the reactant(s) and product(s) may considerably reduce the range of LogP that are available for a particular application; many basically nonpolar molecules possessing some polar structural regions which cause their lack of solubility in strongly hydrophobic solvents. The choice of organic phase will also depend on additional factors such as cost, ease of recovery, fire and fume hazards and specific inhibitory effects. The S-shaped curve can be shifted to the left by immobilising the enzyme within a highly hydrophilic support (Figure 7.2). A simple way of achieving this is to impregnate a beaded hydrophilic polymer (e.g., Sephadex, agarose) with the enzyme followed by suspension of the wet beads directly in the organic phase. These shifts are very important as they greatly increase the choice of suitable organic phase. Such impregnated beads have the additional advantages that
Figure 7.2. Schematic diagram showing the dependence of the activity of immobilised enzymes, in biphasic systems, on the LogP of the organic phase. ——— free enzyme; -------- enzyme immobilised within a strongly hydrophilic support. Biphasic systems may be further stabilised by the use of deuterated water (D2O). This reduces the rate of thermal inactivation, although it does cause an increase in the pKas of ionising groups by about 0.4 with the associated changes in the pH-activity and pH-stability relationships. The higher cost of the deuterated water is offset, to a certain extent, by the small amount necessary in these systems and the ease with which it may be recovered at the end of a catalytic process. Even where the organic solvent has very low LogP and is miscible, the effect of the expected loss in enzymic activity may be offset by changes in the equilibrium constant. Thus it has been proposed that glucose isomerase be used in aqueous ethanol to produce high fructose corn syrup. The equilibrium fraction of fructose can be raised by about 10% to 55% at 30°C in 80% v/v ethanol. This is economically valid even though the enzymic activity drops by about 10% compared to that in the absence of ethanol. This page was established in 2004 and last updated by Martin
Chaplin |
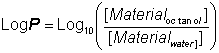 (7.1)
(7.1)