|
|
Glucose from celluloseThere is very much more cellulose available, as a potential source of glucose, than starch, yet cellulose is not a significant source of pure glucose. The reasons for this are many, some technical, some commercial. The fundamental reason is that starch is produced in relatively pure forms by plants for use as an easily biodegradable energy and carbon store. Cellulose is structural and is purposefully combined and associated with lignin and pentosans, so as to resist biodegradation; dead trees take several years to decay even in tropical rainforests. A typical waste cellulolytic material contains less than half cellulose, most of the remainder consisting of roughly equal quantities of lignin and pentosans. A combination of enzymes is needed to degrade this mixture. These enzymes are comparatively unstable of low activity against native lignocellulose and subject to both substrate and product inhibition. Consequently, although many cellulolytic enzymes exist and it is possible to convert pure cellulose to glucose using only enzymes, the cost of this conversion is excessive. The enzymes might be improved by strain selection from the wild or by mutation but problems caused by the physical nature of cellulose are not so amenable to solution. Granular starch is readily stirred in slurries containing 40% (w/v) solids and is easily solubilised but, even when pure, fibrous cellulose forms immovable cakes at 10% solids and remains insoluble in all but the most exotic (and enzyme denaturing) solvents. Impure cellulose often contains almost an equal mass of lignin, which is of little or no value as a by-product and is difficult an expensive to remove. Commercial cellulase preparations from Trichoderma reesei consist of mixtures of the synergistic enzymes:
They are used for the removal of relatively small concentrations of cellulose complexes which have been found to interfere in the processing of plant material in, for example, the brewing and fruit juice industries.
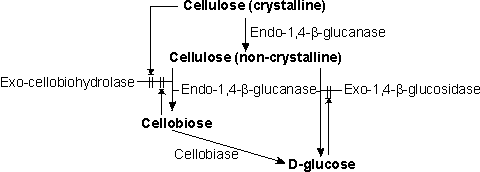
Figure 4.4. Outline of the relationship between the enzyme activities in the hydrolysis of cellulose. || represents inhibitory effects. Endo-1,4-b-glucanase is the rate-controlling activity and may consist of a mixture of enzymes acting on cellulose of different degrees of crystallinity. It acts synergistically with both exo-1,4-b-glucosidase and exo-cellobiohydrolase. Exo-1,4-b-glucosidase is a product-inhibited enzyme. Exo-cellobiohydrolase is product inhibited and additionally appears to be inactivated on binding to the surface of crystalline cellulose. Proper economic analysis reveals that cheap sources of cellulose prove to be generally more expensive as sources of glucose than apparently more expensive starch. Relatively pure cellulose is valuable in its own right, as a paper pulp and chipboard raw material, which currently commands a price of over twice that of corn starch. With the increasing world shortage of pulp it cannot be seen realistically as an alternative source of glucose in the foreseeable future. Knowledge of enzyme systems capable of degrading lignocellulose is advancing rapidly but it is unlikely that lignocellulose will replace starch as a source of glucose syrups for food use. It is, however, quite possible that it may be used, in a process involving the simultaneous use of both enzymes and fermentative yeasts, to produce ethanol; the utilisation of the glucose by the yeast removing its inhibitory effect on the enzymes. It should be noted that cellobiose is a non-fermentable sugar and must be hydrolysed by additional b-glucosidase (EC 3.2.1.21, also called cellobiase for maximum process efficiency (Figure 4.4).
This page was established in 2004 and last updated by Martin
Chaplin |