|
|
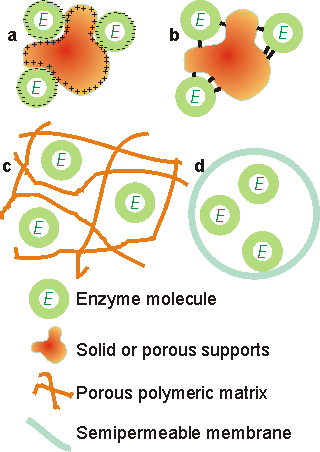
Methods of immobilisationThere are four principal methods available for immobilising enzymes (Figure 3.1):
Figure 3.1. Immobilised enzyme systems. (a) enzyme non-covalently adsorbed to an insoluble particle; (b) enzyme covalently attached to an insoluble particle; (c) enzyme entrapped within an insoluble particle by a cross-linked polymer; (d) enzyme confined within a semipermeable membrane. Carrier matrices for enzyme immobilisation by adsorption and covalent binding must be chosen with care. Of particular relevance to their use in industrial processes is their cost relative to the overall process costs; ideally they should be cheap enough to discard. The manufacture of high-valued products on a small scale may allow the use of relatively expensive supports and immobilisation techniques whereas these would not be economical in the large-scale production of low added-value materials. A substantial saving in costs occurs where the carrier may be regenerated after the useful lifetime of the immobilised enzyme. The surface density of binding sites together with the volumetric surface area sterically available to the enzyme, determine the maximum binding capacity. The actual capacity will be affected by the number of potential coupling sites in the enzyme molecules and the electrostatic charge distribution and surface polarity (i.e., the hydrophobic-hydrophilic balance) on both the enzyme and support. The nature of the support will also have a considerable affect on an enzyme's expressed activity and apparent kinetics. The form, shape, density, porosity, pore size distribution, operational stability and particle size distribution of the supporting matrix will influence the reactor configuration in which the immobilised biocatalyst may be used. The ideal support is cheap, inert, physically strong and stable. It will increase the enzyme specificity (kcat/Km) while reducing product inhibition, shift the pH optimum to the desired value for the process, and discourage microbial growth and non-specific adsorption. Some matrices possess other properties which are useful for particular purposes such as ferromagnetism (e.g., magnetic iron oxide, enabling transfer of the biocatalyst by means of magnetic fields), a catalytic surface (e.g., manganese dioxide, which catalytically removes the inactivating hydrogen peroxide produced by most oxidases), or a reductive surface environment (e.g., titania, for enzymes inactivated by oxidation). Clearly most supports possess only some of these features, but a thorough understanding of the properties of immobilised enzymes does allow suitable engineering of the system to approach these optimal qualities. Adsorption of enzymes onto insoluble supports is a very simple method of wide applicability and capable of high enzyme loading (about one gram per gram of matrix). Simply mixing the enzyme with a suitable adsorbent, under appropriate conditions of pH and ionic strength, followed, after a sufficient incubation period, by washing off loosely bound and unbound enzyme will produce the immobilised enzyme in a directly usable form (Figure 3.2). The driving force causing this binding is usually due to a combination of hydrophobic effects and the formation of several salt links per enzyme molecule. The particular choice of adsorbent depends principally upon minimising leakage of the enzyme during use. Although the physical links between the enzyme molecules and the support are often very strong, they may be reduced by many factors including the introduction of the substrate. Care must be taken that the binding forces are not weakened during use by inappropriate changes in pH or ionic strength. Examples of suitable adsorbents are ion-exchange matrices (Table 3.1), porous carbon, clays, hydrous metal oxides, glasses and polymeric aromatic resins. Ion-exchange matrices, although more expensive than these other supports, may be used economically due to the ease with which they may be regenerated when their bound enzyme has come to the end of its active life; a process which may simply involve washing off the used enzyme with concentrated salt solutions and re-suspending the ion exchanger in a solution of active enzyme.
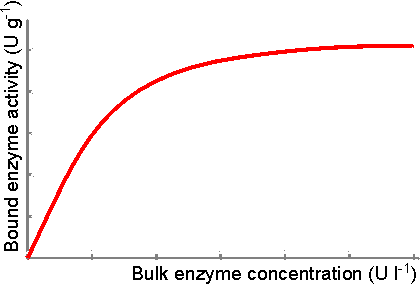
Figure 3.2. Schematic diagram showing the effect of soluble enzyme concentration on the activity of enzyme immobilised by adsorption to a suitable matrix. The amount adsorbed depends on the incubation time, pH, ionic strength, surface area, porosity, and the physical characteristics of both the enzyme and the support. Table 3.1 Preparation of immobilised invertase by adsorption (Woodward 1985)
Immobilisation of enzymes by their covalent coupling to insoluble matrices is an extensively researched technique. Only small amounts of enzymes may be immobilised by this method (about 0.02 gram per gram of matrix) although in exceptional cases as much as 0.3 gram per gram of matrix has been reported. The strength of binding is very strong, however, and very little leakage of enzyme from the support occurs. The relative usefulness of various groups, found in enzymes, for covalent link formation depends upon their availability and reactivity (nucleophilicity), in addition to the stability of the covalent link, once formed (Table 3.2). The reactivity of the protein side-chain nucleophiles is determined by their state of protonation (i.e., charged status) and roughly follows the relationship -S− > -SH > -O− > -NH2 > -COO− > -OH >> -NH3+where the charges may be estimated from a knowledge of the pKa values of the ionising groups (Table 1.1) and the pH of the solution. Lysine residues are found to be the most generally useful groups for covalent bonding of enzymes to insoluble supports due to their widespread surface exposure and high reactivity, especially in slightly alkaline solutions. They also appear to be only very rarely involved in the active sites of enzymes. Table 3.2 Relative usefulness of enzyme residues for covalent coupling
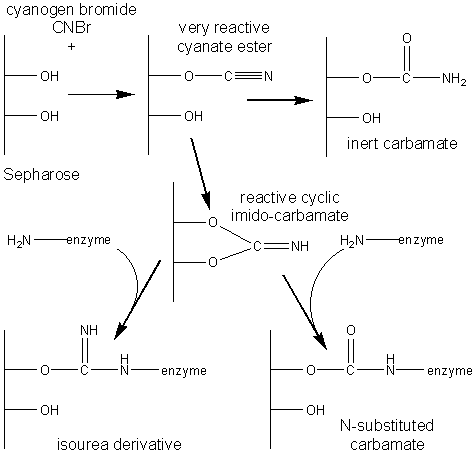
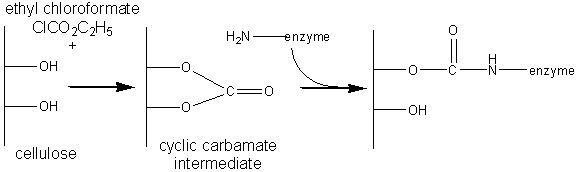
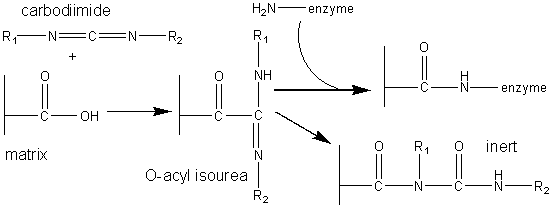
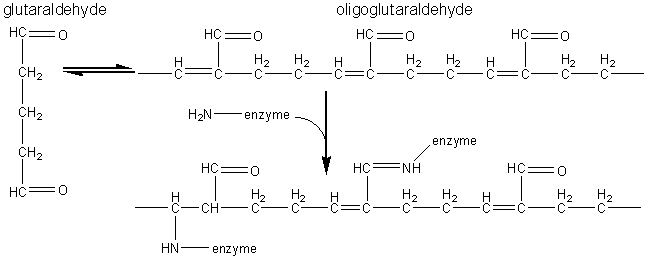
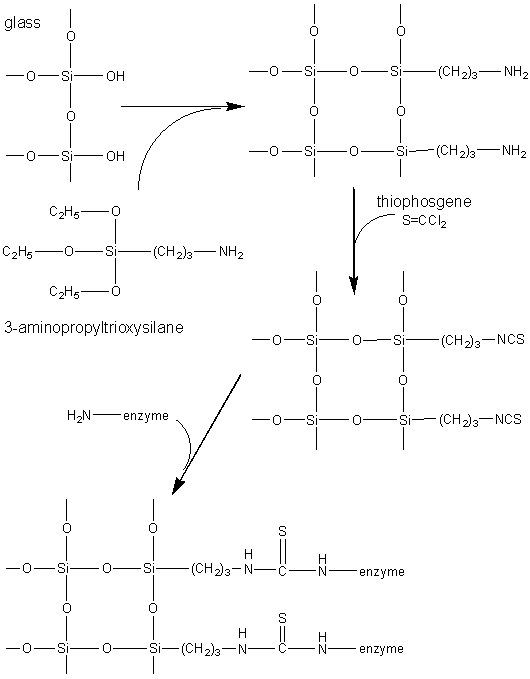
The most commonly used method for immobilising enzymes on the research scale (i.e., using less than a gram of enzyme) involves Sepharose, activated by cyanogen bromide. This is a simple, mild and often successful method of wide applicability. Sepharose is a commercially available beaded polymer which is highly hydrophilic and generally inert to microbiological attack. Chemically it is an agarose (poly-{b-1,3-D-galactose-a-1,4-(3,6-anhydro)-L-galactose}) gel. The hydroxyl groups of this polysaccharide combine with cyanogen bromide to give the reactive cyclic imido-carbonate. This reacts with primary amino groups (i.e. mainly lysine residues) on the enzyme under mildly basic conditions (pH 9 - 11.5, Figure 3.3a). The high toxicity of cyanogen bromide has led to the commercial, if rather expensive, production of ready-activated Sepharose and the investigation of alternative methods, often involving chloroformates, to produce similar intermediates (Figure 3.3b). Carbodiimides (Figure 3.3c) are very useful bifunctional reagents as they allow the coupling of amines to carboxylic acids. Careful control of the reaction conditions and choice of carbodiimide allow a great degree of selectivity in this reaction. Glutaraldehyde is another bifunctional reagent which may be used to cross-link enzymes or link them to supports (Figure 3.3d). It is particularly useful for producing immobilised enzyme membranes, for use in biosensors, by cross-linking the enzyme plus a non-catalytic diluent protein within a porous sheet (e.g., lens tissue paper or nylon net fabric). The use of trialkoxysilanes allows even such apparently inert materials as glass to be coupled to enzymes (Figure 3.3e). There are numerous other methods available for the covalent attachment of enzymes (e.g., the attachment of tyrosine groups through diazo-linkages, and lysine groups through amide formation with acyl chlorides or anhydrides). (a) cyanogen bromide (b) ethyl chloroformate (c) carbodiimide (d) glutaraldehyde (e) 3-aminopropyltriethoxysilane Figure 3.3. Commonly used methods for the covalent immobilisation of enzymes. (a) Activation of Sepharose by cyanogen bromide. Conditions are chosen to minimise the formation of the inert carbamate. (b) Chloroformates may be used to produce similar intermediates to those produced by cyanogen bromide but without its inherent toxicity. (c) Carbodiimides may be used to attach amino groups on the enzyme to carboxylate groups on the support or carboxylate groups on the enzyme to amino groups on the support. Conditions are chosen to minimise the formation of the inert substituted urea. (d) Glutaraldehyde is used to cross-link enzymes or link them to supports. It usually consists of an equilibrium mixture of monomer and oligomers. The product of the condensation of enzyme and glutaraldehyde may be stabilised against dissociation by reduction with sodium borohydride. (e) The use of trialkoxysilane to derivatise glass. The reactive glass may be linked to enzymes by a number of methods including the use thiophosgene, as shown. It is clearly important that the immobilised enzyme retains as much catalytic activity as possible after reaction. This can, in part, be ensured by reducing the amount of enzyme bound in non-catalytic conformations (Figure 3.4). Immobilisation of the enzyme in the presence of saturating concentrations of substrate, product or a competitive inhibitor ensures that the active site remains unreacted during the covalent coupling and reduces the occurrence of binding in unproductive conformations. The activity of the immobilised enzyme is then simply restored by washing the immobilised enzyme to remove these molecules.
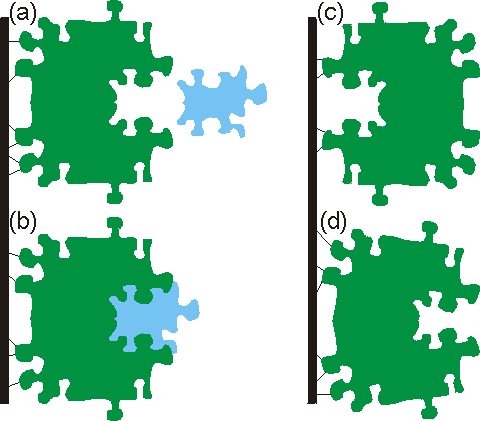
Figure 3.4. The effect of covalent coupling on the expressed activity of an immobilised enzyme. (a) Immobilised enzyme (E) with its active site unchanged and ready to accept the substrate molecule (S), as shown in (b). (c) Enzyme bound in a non-productive mode due to the inaccessibility of the active site. (d) Distortion of the active site produces an inactive immobilised enzyme. Non-productive modes are best prevented by the use of large molecules reversibly bound in or near the active site. Distortion can be prevented by use of molecules which can sit in the active site during the coupling process, or by the use of a freely reversible method for the coupling which encourages binding to the most energetically stable (i.e., native) form of the enzyme. Both (c) and (d) may be reduced by use of 'spacer' groups between the enzyme and support, effectively displacing the enzyme away from the steric influence of the surface. Entrapment of enzymes within gels or fibres is a convenient method for use in processes involving low molecular weight substrates and products. Amounts in excess of 1 g of enzyme per gram of gel or fibre may be entrapped. However, the difficulty which large molecules have in approaching the catalytic sites of entrapped enzymes precludes the use of entrapped enzymes with high molecular weight substrates. The entrapment process may be a purely physical caging or involve covalent binding. As an example of this latter method, the enzymes' surface lysine residues may be derivatised by reaction with acryloyl chloride (CH2=CH-CO-Cl) to give the acryloyl amides. This product may then be copolymerised and cross-linked with acrylamide (CH2=CH-CO-NH2) and bisacrylamide (H2N-CO-CH=CH-CH=CH-CO-NH2) to form a gel. Enzymes may be entrapped in cellulose acetate fibres by, for example, making up an emulsion of the enzyme plus cellulose acetate in methylene chloride, followed by extrusion through a spinneret into a solution of an aqueous precipitant. Entrapment is the method of choice for the immobilisation of microbial, animal and plant cells, where calcium alginate is widely used. Membrane confinement of enzymes may be achieved by a number of quite different methods, all of which depend for their utility on the semipermeable nature of the membrane. This must confine the enzyme while allowing free passage for the reaction products and, in most configurations, the substrates. The simplest of these methods is achieved by placing the enzyme on one side of the semipermeable membrane while the reactant and product stream is present on the other side. Hollow fibre membrane units are available commercially with large surface areas relative to their contained volumes (> 20 m2 L−1) and permeable only to substances of molecular weight substantially less than the enzymes. Although costly, these are very easy to use for a wide variety of enzymes (including regenerating coenzyme systems, see Chapter 8) without the additional research and development costs associated with other immobilisation methods. Enzymes, encapsulated within small membrane-bound droplets or liposomes (see Chapter 7), may also be used within such reactors. As an example of the former, the enzyme is dissolved in an aqueous solution of 1,6-diaminohexane. This is then dispersed in a solution of hexanedioic acid in the immiscible solvent, chloroform. The resultant reaction forms a thin polymeric (Nylon-6,6) shell around the aqueous droplets which traps the enzyme. Liposomes are concentric spheres of lipid membranes, surrounding the soluble enzyme. They are formed by the addition of phospholipid to enzyme solutions. The micro-capsules and liposomes are washed free of non-confined enzyme and transferred back to aqueous solution before use. Table 3.3 presents a comparison of the more important general characteristics of these methods. Table 3.3 Generalised comparison of different enzyme immobilisation techniques.
This page was established in 2004 and last updated by Martin
Chaplin |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||