|
|
What are biosensors?A biosensor is an analytical device which converts a biological response into an electrical signal (Figure 6.1). The term 'biosensor' is often used to cover sensor devices used in order to determine the concentration of substances and other parameters of biological interest even where they do not utilise a biological system directly. This very broad definition is used by some scientific journals (e.g., Biosensors, Elsevier Applied Science) but will not be applied to the coverage here. The emphasis of this Chapter concerns enzymes as the biologically responsive material, but it should be recognised that other biological systems may be utilised by biosensors, for example, whole cell metabolism, ligand binding and the antibody-antigen reaction. Biosensors represent a rapidly expanding field, at the present time, with an estimated 60% annual growth rate; the major impetus coming from the health-care industry (e.g., 6% of the western world are diabetic and would benefit from the availability of a rapid, accurate and simple biosensor for glucose) but with some pressure from other areas, such as food quality appraisal and environmental monitoring. The estimated world analytical market is about £12,000,000,000 year−1 of which 30% is in the health care area. There is clearly a vast market expansion potential as less than 0.1% of this market is currently using biosensors. Research and development in this field is wide and multidisciplinary, spanning biochemistry, bioreactor science, physical chemistry, electrochemistry, electronics and software engineering. Most of this current endeavour concerns potentiometric and amperometric biosensors and colorimetric paper enzyme strips. However, all the main transducer types are likely to be thoroughly examined, for use in biosensors, over the next few years. A successful biosensor must possess at least some of the following beneficial features:
The biological response of the biosensor is determined by the biocatalytic membrane which accomplishes the conversion of reactant to product. Immobilised enzymes possess a number of advantageous features which makes them particularly applicable for use in such systems. They may be re-used, which ensures that the same catalytic activity is present for a series of analyses. This is an important factor in securing reproducible results and avoids the pitfalls associated with the replicate pipetting of free enzyme otherwise necessary in analytical protocols. Many enzymes are intrinsically stabilised by the immobilisation process (see Chapter 3), but even where this does not occur there is usually considerable apparent stabilisation. It is normal to use an excess of the enzyme within the immobilised sensor system. This gives a catalytic redundancy (i.e., h << 1) which is sufficient to ensure an increase in the apparent stabilisation of the immobilised enzyme (see, for example, Figures 3.11, 3.19 and 5.8). Even where there is some inactivation of the immobilised enzyme over a period of time, this inactivation is usually steady and predictable. Any activity decay is easily incorporated into an analytical scheme by regularly interpolating standards between the analyses of unknown samples. For these reasons, many such immobilised enzyme systems are re-usable up to 10,000 times over a period of several months. Clearly, this results in a considerable saving in terms of the enzymes' cost relative to the analytical usage of free soluble enzymes. When the reaction, occurring at the immobilised enzyme membrane of a biosensor, is limited by the rate of external diffusion, the reaction process will possess a number of valuable analytical assets. In particular, it will obey the relationship shown in equation 3.27. It follows that the biocatalyst gives a proportional change in reaction rate in response to the reactant (substrate) concentration over a substantial linear range, several times the intrinsic Km (see Figure 3.12 line e). This is very useful as analyte concentrations are often approximately equal to the Kms of their appropriate enzymes which is roughly 10 times more concentrated than can be normally determined, without dilution, by use of the free enzyme in solution. Also following from equation 3.27 is the independence of the reaction rate with respect to pH, ionic strength, temperature and inhibitors. This simply avoids the tricky problems often encountered due to the variability of real analytical samples (e.g, fermentation broth, blood and urine) and external conditions. Control of biosensor response by the external diffusion of the analyte can be encouraged by the use of permeable membranes between the enzyme and the bulk solution. The thickness of these can be varied with associated effects on the proportionality constant between the substrate concentration and the rate of reaction (i.e., increasing membrane thickness increases the unstirred layer (d) which, in turn, decreases the proportionality constant, kL, in equation 3.27). Even if total dependence on the external diffusional rate is not achieved (or achievable), any increase in the dependence of the reaction rate on external or internal diffusion will cause a reduction in the dependence on the pH, ionic strength, temperature and inhibitor concentrations.
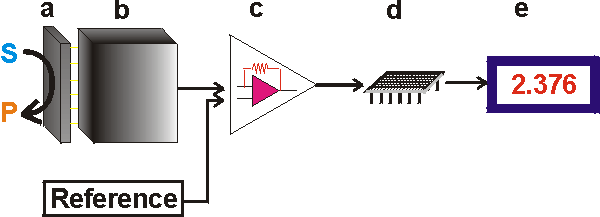
Figure 6.1. Schematic diagram showing the main components of a biosensor. The biocatalyst (a) converts the substrate to product. This reaction is determined by the transducer (b) which converts it to an electrical signal. The output from the transducer is amplified (c), processed (d) and displayed (e). The key part of a biosensor is the transducer (shown as the 'black box' in Figure 6.1) which makes use of a physical change accompanying the reaction. This may be
There are three so-called 'generations' of biosensors; First generation biosensors where the normal product of the reaction diffuses to the transducer and causes the electrical response, second generation biosensors which involve specific 'mediators' between the reaction and the transducer in order to generate improved response, and third generation biosensors where the reaction itself causes the response and no product or mediator diffusion is directly involved. The electrical signal from the transducer is often low and superimposed upon a relatively high and noisy (i.e., containing a high frequency signal component of an apparently random nature, due to electrical interference or generated within the electronic components of the transducer) baseline. The signal processing normally involves subtracting a 'reference' baseline signal, derived from a similar transducer without any biocatalytic membrane, from the sample signal, amplifying the resultant signal difference and electronically filtering (smoothing) out the unwanted signal noise. The relatively slow nature of the biosensor response considerably eases the problem of electrical noise filtration. The analogue signal produced at this stage may be output directly but is usually converted to a digital signal and passed to a microprocessor stage where the data is processed, converted to concentration units and output to a display device or data store.
This page was established in 2004 and last updated by Martin
Chaplin |