|
|
Calorimetric biosensorsMany enzyme catalysed reactions are exothermic, generating heat (Table 6.1) which may be used as a basis for measuring the rate of reaction and, hence, the analyte concentration. This represents the most generally applicable type of biosensor. The temperature changes are usually determined by means of thermistors at the entrance and exit of small packed bed columns containing immobilised enzymes within a constant temperature environment (Figure 6.2). Under such closely controlled conditions, up to 80% of the heat generated in the reaction may be registered as a temperature change in the sample stream. This may be simply calculated from the enthalpy change and the amount reacted. If a 1 mM reactant is completely converted to product in a reaction generating 100 kJ ˣ mol−1 then each ml of solution generates 0.1 J of heat. At 80% efficiency, this will cause a change in temperature of the solution amounting to approximately 0.02°C. This is about the temperature change commonly encountered and necessitates a temperature resolution of 0.0001°C for the biosensor to be generally useful. Table 6.1. Heat output (molar enthalpies) of enzyme catalysed reactions.
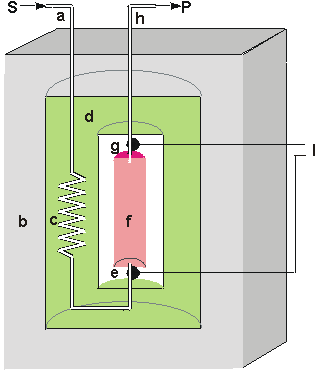
Figure 6.2. Schematic diagram of a calorimetric biosensor. The sample stream (a) passes through the outer insulated box (b) to the heat exchanger (c) within an aluminium block (d). From there, it flows past the reference thermistor (e) and into the packed bed bioreactor (f, 1ml volume), containing the biocatalyst, where the reaction occurs. The change in temperature is determined by the thermistor (g) and the solution passed to waste (h). External electronics (l) determines the difference in the resistance, and hence temperature, between the thermistors. The thermistors, used to detect the temperature change, function by changing their electrical resistance with the temperature, obeying the relationship
therefore:
where R1 and R2 are the resistances of the thermistors at absolute temperatures T1 and T2 respectively and B is a characteristic temperature constant for the thermistor. When the temperature change is very small, as in the present case, B(1/T1) - (1/T2) is very much smaller than one and this relationship may be substantially simplified using the approximation when x<<1 that ex»1 + x (x here being B(1/T1) - (1/T2),
As T1 » T2, they both may be replaced in the denominator by T1.
The relative decrease in the electrical resistance (DR/R) of the thermistor is proportional to the increase in temperature (DT). A typical proportionality constant (-B/T12) is -4%°C−1. The resistance change is converted to a proportional voltage change, using a balanced Wheatstone bridge incorporating precision wire-wound resistors, before amplification. The expectation that there will be a linear correlation between the response and the enzyme activity has been found to be borne out in practice. A major problem with this biosensor is the difficulty encountered in closely matching the characteristic temperature constants of the measurement and reference thermistors. An equal movement of only 1°C in the background temperature of both thermistors commonly causes an apparent change in the relative resistances of the thermistors equivalent to 0.01°C and equal to the full-scale change due to the reaction. It is clearly of great importance that such environmental temperature changes are avoided, which accounts for inclusion of the well-insulated aluminium block in the biosensor design (see Figure 6.2). The sensitivity (10−4 M) and range (10−4 - 10−2 M) of thermistor biosensors are both quite low for the majority of applications although greater sensitivity is possible using the more exothermic reactions (e.g., catalase). The low sensitivity of the system can be increased substantially by increasing the heat output by the reaction. In the simplest case this can be achieved by linking together several reactions in a reaction pathway, all of which contribute to the heat output. Thus the sensitivity of the glucose analysis using glucose oxidase can be more than doubled by the co-immobilisation of catalase within the column reactor in order to disproportionate the hydrogen peroxide produced. An extreme case of this amplification is shown in the following recycle scheme for the detection of ADP.
ADP is the added analyte and excess glucose, phosphoenol pyruvate, NADH and oxygen are present to ensure maximum reaction. Four enzymes (hexokinase, pyruvate kinase, lactate dehydrogenase and lactate oxidase) are co-immobilised within the packed bed reactor. In spite of the positive enthalpy of the pyruvate kinase reaction, the overall process results in a 1000 fold increase in sensitivity, primarily due to the recycling between pyruvate and lactate. Reaction limitation due to low oxygen solubility may be overcome by replacing it with benzoquinone, which is reduced to hydroquinone by flavo-enzymes. Such reaction systems do, however, have the serious disadvantage in that they increase the probability of the occurrence of interference in the determination of the analyte of interest. Reactions involving the generation of hydrogen ions can be made more sensitive by the inclusion of a base having a high heat of protonation. For example, the heat output by the penicillinase reaction may be almost doubled by the use of Tris (tris-(hydroxymethyl)aminomethane) as the buffer.In conclusion, the main advantages of the thermistor biosensor are its general applicability and the possibility for its use on turbid or strongly coloured solutions. The most important disadvantage is the difficulty in ensuring that the temperature of the sample stream remains constant (± 0.01°C).
This page was established in 2004 and last updated by Martin
Chaplin |
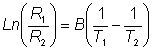 (6.2)
(6.2)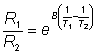 (6.2b)
(6.2b)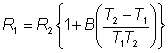 (6.3)
(6.3)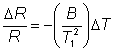 (6.4)
(6.4)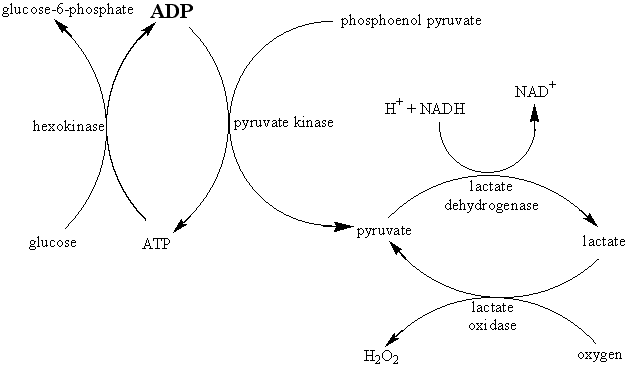 [6.2]
[6.2]