|
|
Centrifugation
where v is the rate of sedimentation, d is the particle diameter, ρs is the particle density, ρl is the solution density, w is the angular velocity in radians s−1, r is the radius of rotation, h is the dynamic viscosity, Fs is a correction factor for particle interaction during hindered settling and q is a shape factor (=1 for spherical particles). Fs depends on the volume fraction of the solids present; approximately equalling 1, 0.5, 0.1 and 0.05 for 1%, 3%, 12% and 20% solids volume fraction respectively. Only material which reaches a surface during the flow through continuous centrifuges will be removed from the centrifuge feedstock, the efficiency depending on the residence time within the centrifuge and the distance necessary for sedimentation (D). This residence time will equal the volumetric throughput (f) divided by the volume of the centrifuge (V). The maximum throughput of a centrifuge for efficient use is given by The efficiency of the process is seen to depend on the solids volume fraction, the effective clarifying surface (V/D) and the acceleration factor (w2r/g, where g is the gravitational constant, 981 cm s−2; a rotor of radius 25 cm spinning at 1 rev s−1 has an acceleration factor of approximately 1 G). Low acceleration factors of about 1 500 g may be used for harvesting cells whereas much higher acceleration factors are needed to collect enzyme efficiently. The product of these factors (w2rV/gD) is called the sigma factor (S) and is used to compare centrifuges and to assist scale-up. Laboratory centrifuges using tubes in swing-out or angle head rotors have high angular velocity (w) and radius of rotation (r) but small capacity (V) and substantial sedimentation distance (D). This type of design cannot be scaled-up safely, primarily because the mechanical stress on the centrifuge head increases with the square of the radius, which must increase with increasing capacity. For large-scale use, continuous centrifuges of various types are employed (Figure 2.2). These allow the continuous addition of feedstock, the continuous removal of supernatant and the discontinuous, semicontinuous or continuous removal of solids. Where discontinuous or semicontinuous removal of precipitate occurs, the precipitate is flushed out by automatic discharge systems which cause its dilution with water or medium and may be a problem if the precipitate is required for further treatment. Centrifugation is the generally preferred method for the collection of enzyme-containing solids as it does not present a great hazard to most enzymes so long as foam production, with consequent enzymic inactivation, is minimised.
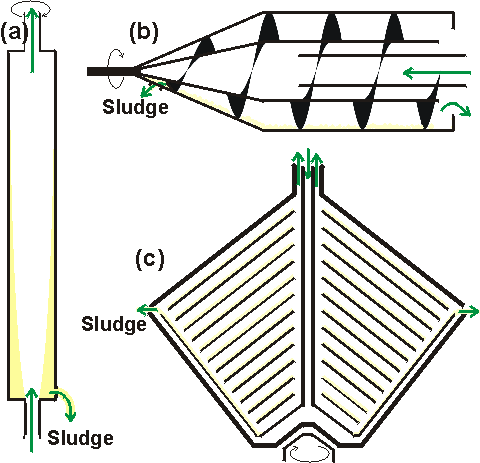
Figure 2.2. Basic designs of industrial centrifuges, showing the flow of material within the bowls. Motor drives, cooling jackets and sludge collection vessels are not shown. (a) Tubular bowl centrifuge. This is generally operated vertically, the tubular rotor providing a long flow path enabling clarification. The sludge collects and must be removed. (b) Continuous scroll centrifuge. This is operated horizontally. The helical screw scrolls the solids along the bowl surface and out of the liquid; the sludge being dewatered before discharge. The clarified liquor overflows over an adjustable weir at the other end of the bowl. The screw conveyer rotates at a slightly different speed to the bowl. (c) Continuous multichamber disc-stack centrifuge. The bowl contains a number of parallel discs providing a large clarifying surface with a small sedimentation distance. The sludge is removed through a valve. Small particles of cell debris and precipitated protein may be sedimented using tubular bowl centrifuges, of which Sharples centrifuges (produced by Pennwalt Ltd.) are the best known. These semi-continuous centrifuges are long and thin enabling rapid acceleration and deceleration, minimising the down-time required for the removal of the sedimented solids. Here the radius and effective liquid thickness are both small allowing a high angular velocity and hence high centrifugal force; small models can be used at acceleration factors up to 50,000 g, accumulating 0.1 Kg of wet deposit whereas large models, designed to accumulate up to 5 Kg of deposit, are restricted to 16,000 g. The capacities of these centrifuges are only moderate.Multichamber disc-stack centrifuges, originally designed (by Westfalia and Alpha-Laval) for cream separation, contain multiple coned discs in a stack which are spun and on which the precipitate collects. They may be operated either semi-continuously or, by using a centripetal pressurising pump within the centrifuge bowl which forces the sludge out through a valve, continuously. The capacity and radius of such devices are large and the thickness of liquid is very small, due to the large effective surface area. The angular velocity, however, is restricted giving a maximum acceleration factor of about 8,000 g. A different design which is rather similar in principle is the solid bowl scroll centrifuge in which an Archimedes' screw collects the precipitate so that fluid and solids leave at opposite ends of the apparatus. These can only be used at low acceleration (about 3,000 g) so they are suitable only for the collection of comparatively large particles. Although many types of centrifuge are available, the efficient precipitation of small particles of cell debris can be difficult, sometimes near-impossible. Clearly from Equation 2.2, the efficiency of centrifugation can be improved if the particle diameter (d) is increased. This can be done either by coagulating or flocculating particles. Coagulation is caused by the removal of electrostatic charges (e.g., by pH change) and allowing particles to adhere to each other. Flocculation is achieved by adding small amounts of high-molecular-weight charged materials which bridge oppositely-charged particles to produce a loose aggregate which may be readily removed by centrifugation or filtration. Flocculation and coagulation are cheap and effective aids to precipitating or otherwise harvesting whole cells, cell debris or soluble proteins but, of course, it is essential that the agents used must not inhibit the target enzymes. It is important to note that the choice of flocculant is determined by the pH and ionic strength of the solution and the nature of the particles. Most flocculants have very definite optimum concentrations above which further addition may be counter-effective. Some flocculants can be rapidly ruined by shear. A comparatively recent introduction designed for the removal of cell debris is a moderately hydrophobic product in which cellulose is lightly derivatised with diethylaminoethyl functional groups. This material (Whatman CDR; cell debris remover) is inexpensive (essential as it is not reusable), binds to unwanted negatively charged cell constituents, acts as a filter aid and may be incinerated to dispose of hazardous wastes.
This page was established in 2004 and last updated by Martin
Chaplin |