|
|
Preparation of enzymesReaders of papers dealing with the preparation of enzymes for research purposes will be familiar with tables detailing the stages of purification. Often the enzyme may be purified several hundred-fold but the yield of the enzyme may be very poor, frequently below 10% of the activity of the original material (Table 2.2). In contrast, industrial enzymes will be purified as little as possible, only other enzymes and material likely to interfere with the process which the enzyme is to catalyse, will be removed. Unnecessary purification will be avoided as each additional stage is costly in terms of equipment, manpower and loss of enzyme activity. As a result, some commercial enzyme preparations consist essentially of concentrated fermentation broth, plus additives to stabilise the enzyme's activity. Table 2.2. The effect of number of steps on the yield and costs in a typical enzyme purification process. The realistic assumptions are made that step yields are 75%, step purifications are three-fold and step costs are 10% of the initial costs (later purification steps are usually intrinsically more expensive but are necessarily of smaller scale).
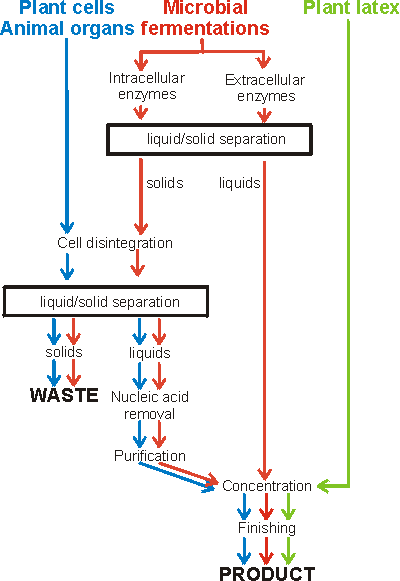
The content of the required enzyme should be as high as possible (e.g., 10% w/w of the protein) in order to ease the downstream processing task. This may be achieved by developing the fermentation conditions or, often more dramatically, by genetic engineering. It may well be economically viable to spend some time cloning extra copies of the required gene together with a powerful promoter back into the producing organism in order to get 'over-producers' (see Chapter 8). It is important that the maximum activity is retained during the preparation of enzymes. Enzyme inactivation can be caused by heat, proteolysis, sub-optimal pH, oxidation, denaturants, irreversible inhibitors and loss of cofactors or coenzymes. Of these heat inactivation, which together with associated pH effects, is probably the most significant. It is likely to occur during enzyme extraction and purification if insufficient cooling is available (see Chapter 1), but the problem is less when preparing thermophilic enzymes. Proteolysis is most likely to occur in the early stages of extraction and purification when the proteases responsible for protein turnover in living cells are still present. It is also the major reason for enzyme inactivation by microbial contamination. In their native conformations, enzymes have highly structured domains which are resistant to attack by proteases because many of the peptide bonds are mechanically inaccessible and because many proteases are highly specific. The chances of a susceptible peptide bond in a structured domain being available for protease attack are low. Single 'nicks' by proteases in these circumstances may have little immediate effect on protein conformation and, therefore, activity. The effect, however, may severely reduce the conformational stability of the enzyme to heat or pH variation so greatly reducing its operational stability. If the domain is unfolded under these changed conditions, the whole polypeptide chain may be available for proteolysis and the same, specific, protease may destroy it. Clearly the best way of preventing proteolysis is to rapidly remove, or inhibit, protease activity. Before this can be achieved it is important to keep enzyme preparations cold to maintain their native conformation and slow any protease action that may occur. Some intracellular enzymes are used commercially without isolation and purification but the majority of commercial enzymes are either produced extracellularly by the microbe or plant or must be released from the cells into solution and further processed (Figure 2.1). Solid/liquid separation is generally required for the initial separation of cell mass, the removal of cell debris after cell breakage and the collection of precipitates. This can be achieved by filtration, centrifugation or aqueous biphasic partition. In general, filtration or aqueous biphasic systems are used to remove unwanted cells or cell debris whereas centrifugation is the preferred method for the collection of required solid material. Figure 2.1. Flow diagram for the preparation of enzymes.
This page was established in 2004 and last updated by Martin
Chaplin |