|
|
Production of glucose syrupThe liquefied starch at 8 -12 DE is suitable for saccharification to produce syrups with DE values of from 45 to 98 or more. The greatest quantities produced are the syrups with DE values of about 97. At present these are produced using the exoamylase, glucan 1,4-a-glucosidase (1,4-a-D-glucan glucohydrolase, commonly called glucoamylase but also called amyloglucosidase and g-amylase), which releases b-D-glucose from 1,4-a-, 1,6-a- and 1,3-a-linked glucans. In theory, carefully liquefied starch at 8 -12 DE can be hydrolysed completely to produce a final glucoamylase reaction mixture with DE of 100 but, in practice, this can be achieved only at comparatively low substrate concentrations. The cost of concentrating the product by evaporation decrees that a substrate concentration of 30% is used. It follows that the maximum DE attainable is 96 - 98 with syrup composition 95 - 97% glucose, 1 - 2% maltose and 0.5 - 2% (w/w) isomaltose (a-D-glucopyranosyl-(1,6)-D-glucose). This material is used after concentration, directly for the production of high-fructose syrups or for the production of crystalline glucose. Whereas liquefaction is usually a continuous process, saccharification is most often conducted as a batch process. The glucoamylase most often used is produced by Aspergillus niger strains. This has a pH optimum of 4.0 - 4.5 and operates most effectively at 60°C, so liquefied starch must be cooled and its pH adjusted before addition of the glucoamylase. The cooling must b rapid, to avoid retrogradation (the formation of intractable insoluble aggregates of amylose; the process that gives rise to the skin on custard). Any remaining bacterial a-amylase will be inactivated when the pH is lowered; however, this may be replaced later by some acid-stable a-amylase which is normally present in the glucoamylase preparations. When conditions are correct the glucoamylase is added, usually at the dosage of 0.65 - 0.80 litre enzyme preparation.tonne−1 starch (200 U kg−1). Saccharification is normally conducted in vast stirred tanks, which may take several hours to fill (and empty), so time will be wasted if the enzyme is added only when the reactors are full. The alternatives are to meter the enzyme at a fixed ratio or to add the whole dose of enzyme at the commencement of the filling stage. The latter should give the most economical use of the enzyme.
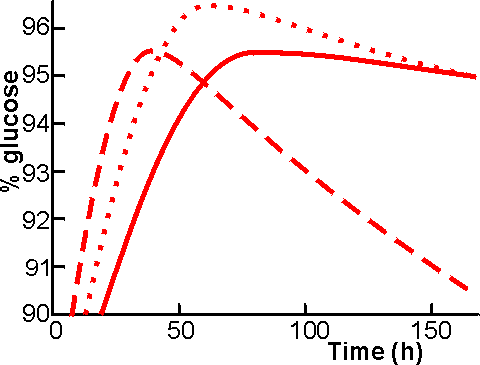
Figure 4.3. The % glucose formed from 30% (w/w) 12 DE maltodextrin, at 60°C and pH 4.3, using various enzyme solutions. ———200 U kg−1 Aspergillus niger glucoamylase; -----------400 U kg−1 A. niger glucoamylase; ········· 200 U kg−1 A. niger glucoamylase plus 200 U kg−1 Bacillus acidopullulyticus pullulanase. The relative improvement on the addition of pullulanase is even greater at higher substrate concentrations. The saccharification process takes about 72 h to complete but may, of course, be speeded up by the use of more enzyme. Continuous saccharification is possible and practicable if at least six tanks are used in series. It is necessary to stop the reaction, by heating to 85°C for 5 min, when a maximum DE has been attained. Further incubation will result in a fall in the DE, to about 90 DE eventually, caused by the formation of isomaltose as accumulated glucose re-polymerises with the approach of thermodynamic equilibrium (Figure 4.3). The saccharified syrup is filtered to remove fat and denatured protein released from the starch granules and may then be purified by passage through activated charcoal and ion-exchange resins. It should be remembered that the dry substance concentration increases by about 11 % during saccharification, because one molecule of water is taken up for each glycosidic bond hydrolysed (molecule of glucose produced). Although glucoamylase catalyzes the hydrolysis of 1,6-a-linkages, their breakdown is slow compared with that of 1,4-a-linkages (e.g., the rates of hydrolysing the 1,4-a, 1,6-a and 1,3-a-links in tetrasaccharides are in the proportions 300 : 6 : 1). It is clear that the use of a debranching enzyme would speed the overall saccharification process but, for industrial use such an enzyme must be compatible with glucoamylase. Two types of debranching enzymes are available: pullulanase, which acts as an exo hydrolase on starch dextrins; and isoamylase (EC.3.2.1.68), which is a true endohydrolase. Novo Industri A/S have recently introduced a suitable pullulanase, produced by a strain of Bacillus acidopullulyticus. The pullulanase from Klebsiella aerogenes which has been available commercially to some time is unstable at temperatures over 45°C but the B. acidopullulyticus enzymes can be used under the same conditions as the Aspergillus glucoamylase (60°C, pH 4.0-4.5). The practical advantage of using pullulanase together with glucoamylase is that less glucoamylase need be used This does not in itself give any cost advantage but because less glucoamylase is used and fewer branched oligosaccharides accumulate toward the end of the saccharification, the point at which isomaltose production becomes significant occurs at higher DE (Figure 4.3). It follows that higher DE values and glucose contents can be achieved when pullulanase is use (98 - 99 DE and 95 - 97% (w/w) glucose, rather than 97 - 98 DE) and higher substrate concentrations (30 - 40% dry solids rather than 25 - 30%) may be treated. The extra cost of using pullulanase is recouped by savings in evaporation and glucoamylase costs. In addition, when the product is to be used to manufacture high-fructose syrups, there is a saving in the cost of further processing. The development of the B. acidopullulyticus pullulanase is an excellent example of what can be done if sufficient commercial pull exists for a new enzyme. The development of a suitable a-D-glucosidase, in order to reduce the reversion, would be an equally useful step for industrial glucose production. Screening of new strains of bacteria for a novel enzyme of this type is a major undertaking. It is not surprising that more details of the screening procedures used are not readily available.
This page was established in 2004 and last updated by Martin
Chaplin |