|
|
The use of enzymes in starch hydrolysisStarch is the commonest storage carbohydrate in plants. It is used by the plants themselves, by microbes and by higher organisms so there is a great diversity of enzymes able to catalyse its hydrolysis. Starch from all plant sources occurs in the form of granules which differ markedly in size and physical characteristics from species to species. Chemical differences are less marked. The major difference is the ratio of amylose to amylopectin; e.g., corn starch from waxy maize contains only 2% amylose but that from amylomaize is about 80% amylose. Some starches, for instance from potato, contain covalently bound phosphate in small amounts (0.2% approximately), which has significant effects on the physical properties of the starch but does not interfere with its hydrolysis. Acid hydrolysis of starch has had widespread use in the past. It is now largely replaced by enzymic processes, as it required the use of corrosion resistant materials, gave rise to high colour and saltash content (after neutralisation), needed more energy for heating and was relatively difficult to control.
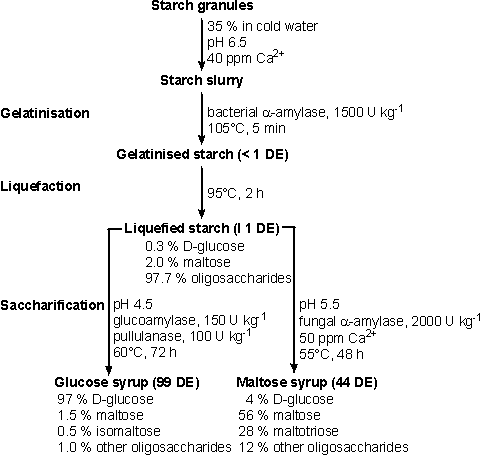
Figure 4.2. The use of enzymes in processing starch. Typical conditions are given. Of the two components of starch, amylopectin presents the great challenge to hydrolytic enzyme systems. This is due to the residues involved in a-1,6-glycosidic branch points which constitute about 4 - 6% of the glucose present. Most hydrolytic enzymes are specific for a-1,4-glucosidic links yet the a-1,6-glucosidic links must also be cleaved for complete hydrolysis of amylopectin to glucose. Some of the most impressive recent exercises in the development of new enzymes have concerned debranching enzymes. It is necessary to hydrolyse starch in a wide variety of processes which m be condensed into two basic classes:
In the former processes, such as glucose syrup production, starch is usually the major component of reaction mixtures, whereas in the latter processes, such as the processing of sugar cane juice, small amounts of starch which contaminate non-starchy materials are removed. Enzymes of various types are used in these processes. Although starches from diverse plants may be utilised, corn is the world's most abundant source and provides most of the substrate used in the preparation of starch hydrolysates. There are three stages in the conversion of starch (Figure 4.2):
Gelatinisation is achieved by heating starch with water, and occurs necessarily and naturally when starchy foods are cooked. Gelatinised starch is readily liquefied by partial hydrolysis with enzymes or acids and saccharified by further acidic or enzymic hydrolysis. The starch and glucose syrup industry uses the expression dextrose equivalent or DE, similar in definition to the DH units of proteolysis, to describe its products, where:
In practice, this is usually determined analytically by use of the closely related, but not identical, expression: Thus, DE represents the percentage hydrolysis of the glycosidic linkages present. Pure glucose has a DE of 100, pure maltose has a DE of about 50 (depending upon the analytical methods used; see equation (4.3)) and starch has a DE of effectively zero. During starch hydrolysis, DE indicates the extent to which the starch has been cleaved. Acid hydrolysis of starch has long been used to produce 'glucose syrups' and even crystalline glucose (dextrose monohydrate). Very considerable amounts of 42 DE syrups are produced using acid and are used in many applications in confectionery. Further hydrolysis using acid is not satisfactory because of undesirably coloured and flavoured breakdown products. Acid hydrolysis appears to be a totally random process which is not influenced by the presence of a-1,6-glucosidic linkages. Table 4.2 Enzymes used in starch hydrolysis
The nomenclature of the enzymes used commercially for starch hydrolysis is somewhat confusing and the EC numbers sometimes lump together enzymes with subtly different activities (Table 4.2). For example, a-amylase may be subclassified as liquefying or saccharifying amylases but even this classification is inadequate to encompass all the enzymes that are used in commercial starch hydrolysis. One reason for the confusion in the nomenclature is the use of the anomeric form of the released reducing group in the product rather than that of the bond being hydrolysed; the products of bacterial and fungal a-amylases are in the a-configuration and the products of b-amylases are in the b-configuration, although all these enzymes cleave between a-1,4-linked glucose residues. The a-amylases (1,4-a-D-glucan glucanohydrolases) are endohydrolases which cleave 1,4-a-D-glucosidic bonds and can bypass but cannot hydrolyse 1,6-a-D-glucosidic branchpoints. Commercial enzymes used for the industrial hydrolysis of starch are produced by Bacillus amyloliquefaciens (supplied by various manufacturers) and by B. licheniformis (supplied by Novo Industri A/S as Termamyl). They differ principally in their tolerance of high temperatures, Termamyl retaining more activity at up to 110°C, in the presence of starch, than the B. amyloliquefaciens a-amylase. The maximum DE obtainable using bacterial a-amylases is around 40 but prolonged treatment leads to the formation of maltulose (4-a-D-glucopyranosyl-D-fructose), which is resistant to hydrolysis by glucoamylase and a-amylases. DE values of 8-12 are used in most commercial processes where further saccharification is to occur. The principal requirement for liquefaction to this extent is to reduce the viscosity of the gelatinised starch to ease subsequent processing. Various manufacturers use different approaches to starch liquefaction using a-amylases but the principles are the same. Granular starch is slurried at 30-40% (w/w) with cold water, at pH 6.0-6.5, containing 20-80 ppm Ca2+ (which stabilises and activates the enzyme) and the enzyme is added (via a metering pump). The a-amylase is usually supplied at high activities so that the enzyme dose is 0.5-0.6 kg tonne−1 (about 1500 U kg−1 dry matter) of starch. When Termamyl is used, the slurry of starch plus enzyme is pumped continuously through a jet cooker, which is heated to 105°C using live steam. Gelatinisation occurs very rapidly and the enzymic activity, combined with the significant shear forces, begins the hydrolysis. The residence time in the jet cooker is very brief. The partly gelatinised starch is passed into a series of holding tubes maintained at 100-105°C and held for 5 min to complete the gelatinisation process. Hydrolysis to the required DE is completed in holding tanks at 90-100°C for 1 to 2 h. These tanks contain baffles to discourage backmixing. Similar processes may be used with B. amyloliquefaciens a-amylase but the maximum temperature of 95°C must not be exceeded. This has the drawback that a final 'cooking' stage must be introduced when the required DE has been attained in order to gelatinise the recalcitrant starch grains present in some types of starch which would otherwise cause cloudiness in solutions of the final product. The liquefied starch is usually saccharified but comparatively small amounts are spray-dried for sale as 'maltodextrins' to the food industry mainly for use as bulking agents and in baby food. In this case, residual enzymic activity may be destroyed by lowering the pH towards the end of the heating period. Fungal a-amylase also finds use in the baking industry. It often needs to be added to bread-making flours to promote adequate gas production and starch modification during fermentation. This has become necessary since the introduction of combine harvesters. They reduce the time between cutting and threshing of the wheat, which previously was sufficient to allow a limited sprouting so increasing the amounts of endogenous enzymes. The fungal enzymes are used rather than those from bacteria as their action is easier to control due to their relative heat lability, denaturing rapidly during baking.
This page was established in 2004 and last updated by Martin
Chaplin |