
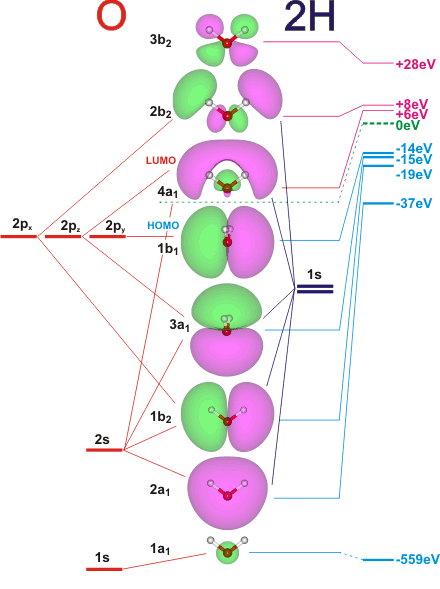
The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a1)2(2a1)2(1b2)2(3a1)2(1b1)2(4a1)0(2b2)0(3b2)0 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. b (experimental data is given in [1289]). They are set out with the lowest energy (that is, most negative energy) molecular orbitals at the bottom. They are all given in the xz plane (z-axis upwards) except 1b1 and 3a1, which are in the yz plane (z-axis upwards).a The two lowest energy orbitals 1a1(non-bonding) and 2a1 (bonding) are contributed from the 1s (completely) and 2s (mostly) orbitals of the oxygen atom, respectively, and are consequentially approximately spherical. The three highest-energy occupied orbitals (1b2 bonding, 3a1 non-bonding, 1b1 non-bonding) are orthogonal around the oxygen atom and without obvious sp3 hybridization characteristics.
The relative energies of these orbitals have been found to be somewhat different from these theoretical values. The lowest energy transitions are broad at 7.61 and 9.36 eV for the 3s/4a1←1b1 and 3s/4a1←3a1 transitions, respectively [1561] for the gas phase and at 8.09 and 9.74 eV in the liquid [1561, 1562].
The highest occupied molecular orbital (HOMO), 1b1, is predominantly pz2 in character with no contribution from the hydrogen 1s orbital and mainly contributes to the "lone pair" effects. The 2a1, 1b2 and 3a1 all contribute to the O-H bonds. The lowest unoccupied molecular orbitals 4a1 (LUMO) and 2b2 are O-H antibonding orbitals, seen in X-ray spectroscopy. They have the greatest electron densities around the O-atom, whereas orbital 3b2 has the greatest electron density around the H-atoms. The larger the HOMO-LUMO gap, the higher the chemical stability. Overall, these unoccupied orbitals take up more space. The experimental binding energy of the 1a1 orbital in the gas phase is 539.9 eV [1227].
These orbitals are appreciably changed in ice and water; the experimental (averaged) electron binding energies in liquid water being 2a1 30.90 eV, 1b2 17.34 eV, 3a1 13.50 eV, 1b1 11.16 eV [877]. The experimental binding energy of the 1a1 orbital in the liquid phase consists of a broad energy distribution centered about 538.1 eV plus a smaller contribution at 536.6 eV within the tetrahedrally hydrogen-bonded bulk [1227]. The 1b2 and 3a1 orbitals are largely responsible for the donation of hydrogen bonding, with the 3a1 orbital shown experimentally to contribute the most [411]. Also, the 4a1 and 2b2 antibonding orbitals are reported to be partially occupied in hydrogen bond formation, receiving electron density from donor 1b1 orbitals [814].
The potentials of the ground and core-excited (4a1, 2b2 and 2b1) states of gas-phase water have been described [2906]
An interactive structure with orbitals is available (COW [Plug-in, ActiveX] only).
Water symmetry
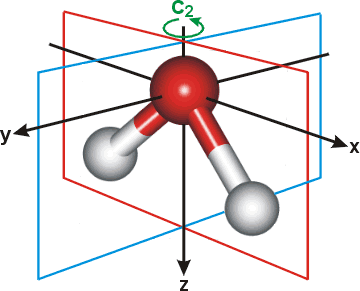
a The nomenclature is based on the symmetry of the orbitals. The figure right shows the planes of symmetry (xz and yz) and the two-fold axis of rotation (C2, z-axis).
If the orbitals are unchanged (that is, symmetric) with respect to the two-fold axis of rotation (C2 = 180° rotation), then they are labeled 'a', otherwise the label is ‘b’ (for anti-symmetric). If they are symmetric with respect to the (xz) plane of symmetry, an additional label 1 is specified, leading to a1 or b1 orbitals. Otherwise the label is 2 which leads to a2 and b2 orbitals. For example the b1 orbital is anti-symmetric with respect to the C2 rotation and symmetric with respect to the xz plane. For a deeper symmetry reason, b1 orbitals are always anti-symmetric with respect to the molecular plane (yz). The orbitals are then numbered from the lowest energy (i.e., 1a1 is the lowest energy a1 orbital, 2a1 is the next one). [Back]
b The 6-31G** basis set. Orbitals are represented by 6 Gaussian functions near the nucleus and 3 away from the nucleus with one Gaussian for hydrogen atoms. Polarization is by means of d-functions on first row atoms, including hydrogens. [Back]
Home | Site Index | Water molecule | Water dimer molecular orbitals | LSBU | Top
This page was established in 2001 and last updated by Martin Chaplin on 5 August, 2021