
Molybdenum Blue crystals

![]() Structure of the Mo154 nanowheel
Structure of the Mo154 nanowheel
![]() How does the {Mo154} nanowheel hydrate?
How does the {Mo154} nanowheel hydrate?
![]() How do the 1165 hydrated {Mo154} wheels form large spherical clusters?
How do the 1165 hydrated {Mo154} wheels form large spherical clusters?
Molybdates in aqueous solution can form a vast variety of structures due to the flexibility in the Mo-O-Mo links, the accessibleredox changes particularly involving MoV and MoVI, the different Mo coordination numbers possible, particularly 6 and 7, the strong hydration stabilization, and the formation of Mo=O chain termination [1179].
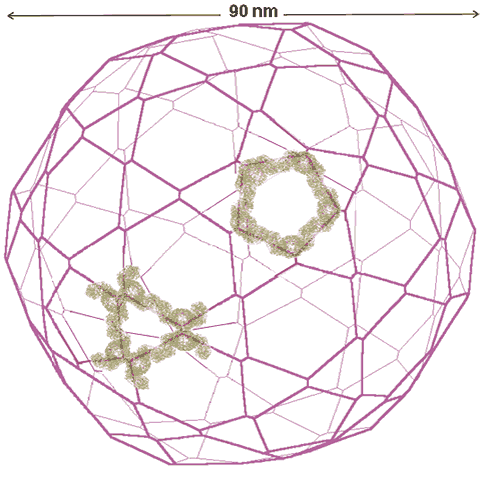
It has been reported that structured (tetrahedral order with strong hydrogen bonds) interstitial water can aid the self-assembly of vesicles [4279]. This was proposed in 2007 for the self-assembly of polyoxomolybdate nanostructures. This centered around the self-assembling molybdate nanostructures; the Mo132 nanocapsule, mentioned elsewhere, and the 'molybdenum blue' {Mo154} nanowheels ([(Mo)Mo5Mo5O33.(H2O)5]14.(H2O)n) shown below and easily obtained from the well-defined, crystalline parent compound Na15[MoVI126MoV28O462H14(H2O)70]0.5[MoVI124MoV28O457H14(H2O)68]0.5≈ 400 H2O [1182] (see top right). They are torus-shaped with highly hydrophilic surfaces.
The structure of the Mo154 nanowheel, hydration centers are shown in yellow on the right
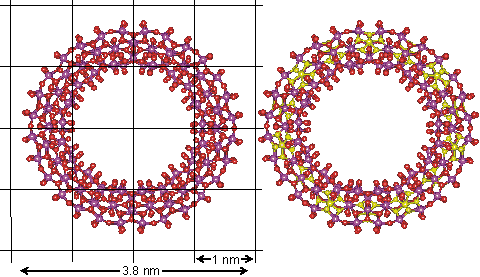
This structure is notable as it has seven-fold symmetry. The torus‘s outer surface to outer surface diameter is about 3.8 nm, with just under half taken up by the central cavity. There are fourteen pentagonal (Mo)Mo5 groups (highlighted in yellow on the right), alternating with seven on each side of the wheel. The interactive structure of this cluster is available (Jmol). The {Mo154} nanowheels hydrate strongly on all surfaces to the surrounding solvent water due to the 70 surface H2O ligands and the large number of other surface oxygen atoms having high electron densities as the 14 (Mo)Mo5Mo5O33 clusters each contain two delocalized 4d electrons, so also giving rise to the characteristic intense 'molybdenum blue' color [1182].
Nanowheel growth to give giant spherical shell structure
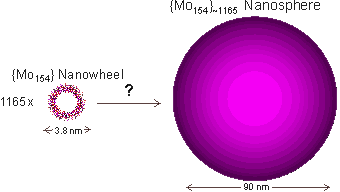
Over days, the {Mo154} nanowheels form very large, essentially monodisperse, blue spherical shells. These are perhaps the largest known self-assembling inorganic clusters. As the radius of gyration equals the hydrodynamic radius for these spheres, they must consist of hollow spheres. Scanning electron microscope images and light scattering data show the radius and mass of the nanospheres is about 45.2±1.4 nm with about 1165 {Mo154} clusters on the nanosphere surface, leaving >50% extra space between the {Mo154} nanowheels if evenly spread [1180]. Thus the {Mo154} clusters may only contact each other through the interfacial water network. a
This is a clear, if unusual, example of how a relatively small amount of interfacial water can organize extensive volumes of liquid water. The question remains
how 1165 such units with unusual seven-fold symmetry can form such highly ordered self-assembling structures.
A putative model must show a rational final structure and use a realizable route from the monomer to the complete cluster in agreement with the data. b [Back to Top ![]() ]
]
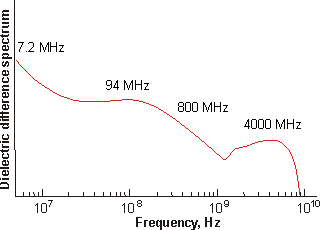
The clustering begins with the hydration of the {Mo154} nanowheels. This has been investigated using conductance-corrected dielectric difference spectra of the freshly prepared 1.82 mM solution [1181]. In the spectrum (below) the wing of the intense absorption near 20 GHz and due to bulk water has been subtracted [1181].
Dielectric difference spectra of a fresh 1.82 mM {Mo154}
nanowheel solution, from [1181]
| Water type |
Assigned relaxation time, ns |
Amplitude, H2 O |
Amplitude,
{Mo154} |
|---|---|---|---|
| 1 |
22 (7.2 MHz) |
- |
7.1 |
| 2 |
1.7 (94 MHz) |
- |
4.4 |
| 3 |
0.2 (800 MHz) |
- |
2.6 |
| 4 |
0.04 (4000 MHz) |
- |
2.8 |
| Bulk |
0.00822 (19400 MHz) |
73.1 |
68.0 |
The amplitudes are proportional to the number density of the dipoles and the square of their dipole values in the bulk state. Due to their symmetry, the {Mo154} nanowheels and larger vesicles do not possess a net dipole moment, thus these amplitudes are due solely to the water molecules. Although the totality of the dielectric amplitude data has increased from 73.1 to 84.9, this may be accommodated simply by a 10% dipole increase of the bulk water, due to their increased polarization on clustering or, if spread out over all the water molecules, the dipole increase need be under 8%. Thus ≈ 20% of the water in this 1.82 mM solution is associated with the {Mo154} nanowheels, ≈ 30% lies inside the spherical nanoshells (as calculated from their volume) and 50% is bulk water.
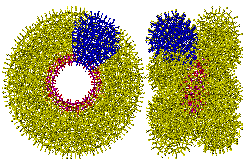
It is reasonable to assume that the hydration close to the surface of the 14 pentagonal (Mo)Mo5 groups in the {Mo154} nanowheels will be similar to that known to exist close to the 12 pentagonal (Mo)Mo5 groups in the {Mo132} nanocapsule.
The link to water (O atoms are shown green)
around the water nanowheel
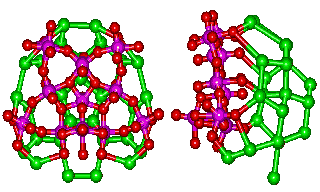
However, the {Mo154} nanowheel (Mo)Mo5 groups (Mo and O atoms shown magenta and red respectively) are surrounded by extra 'free' oxomolybdate, which triple the number of potential anchor points from 5 to 15 (to H2O, shown green) without straining the hydrogen-bonding. Such an arrangement allows icosahedral clusters (ES) to be formed at each of the 14 such anchor points around the perimeter of the {Mo154} nanowheel. The putative growth of this clustering is visualized below.
The {Mo154} nanowheel hydration;
press Forward or Backward
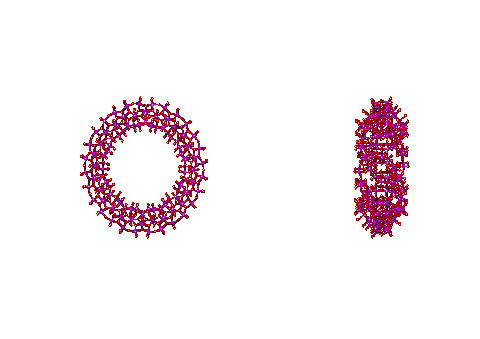
Only the oxygen atoms of the water network are shown yellow above.
The hydration of the {Mo154} nanowheel is outlined opposite (press the Forward button), indicating the layers of hydration, although they are not thought to build up exactly as shown.
Below is shown (blue) the hydration associated with one of the 14 (Mo)Mo5 anchor points.
One of the 14 sites
Shown below is the (H2O)376 water cluster arrangement held by each one of the 14 (Mo)Mo5 anchor points, compared with the arrangement of the (H2O)400 water cluster in the icosahedral water cluster (ES); only the O-atoms of water are shown, yellow.
Three layers of hydration around the {Mo154} nanowheel are indicated below in green, blue, and yellow. There are 14 sites with clear potential for binding cations such as Na+, indicated below by mousing over the image. These sites lie on the inner surface of the nanowheels rather than their highly hydrated outer surface, where they would necessarily disrupt the hydrogen bonding.
![The three layers of hydration around the {Mo154} nanowheel shown green, blue and yellow, with 14 potential Na+ binding sites]](images/giantc.gif)
If the arrangement of water around {Mo154} nanowheels is similar to the icosahedral clustering established earlier, then the hydration falls into three distinct layers.
The three layers of water around {Mo154} nanowheels
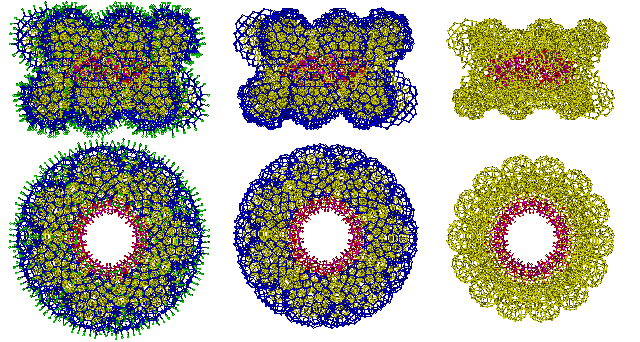
Cartoon showing the hydration assignment involving icosahedral clustering around each (Mo)Mo5 center, ignoring the hydration space in the middle of the torus. There are 980 loosely-held (hydrogen-bonded), H2O (O-atoms are shown green above) either singly-linked or doubly-linked on the outside of the cluster. Underneath lies 1778 still triply-linked (after removal of the loosely held water) then singly-linked (after removal of the triply-linked) H2O (both shown blue). Closest to the nanowheel is an inner layer of 2506 still triply- and tetra-linked (after removal of the outer two layers) H2O (shown yellow).
The numbers of molecules in the hydration layers fit neatly with the results from the dielectric absorption spectra:
Water type |
Relaxation time, ns |
Amplitude |
Experimental, % H2O (H2O/Mo154) |
Calculated from the model, % H2O (H2O/Mo154) |
|---|---|---|---|---|
| 1 |
22 |
7.1 |
8.3 (2505) |
8.4 (2506) |
| 2 |
1.7 |
4.4 |
5.2 (1552) |
5.9 (1778) |
| 3 |
0.2 |
2.6 |
3.1 (917) |
2.7 (798) |
| 4 |
0.04 |
2.8 |
3.3 (988) |
3.3 (980) |
| Bulk |
0.00822 |
68 |
80.1 |
79.8 |
where type 1 water constitutes the inner water shell (shown yellow above), type 2 water constitutes the middle water shell (shown blue above), type 3 water constitutes the water within the central cavity (calculated using its volume and the normal density of water) and type 4 water constitutes the outer water shell (shown green above). There are about 29950 moles of water per mole of {Mo154} nanowheel present,of which ≈ 20% is associated, representing 5962 molecules of H2O. The model, which has been developed independent of this dielectric data, indicates 6062 molecules of H2O (<2% error) with a remarkably good fit with the occupancy of the layers considering the precision of the dielectric data. The type 3 water is the most error-prone of all the assignments as the extent of the central space within the torus is poorly bounded top and bottom but here estimated from the content of a cylindrical volume bounded by the strong type 1, and type 2 water clustering only. Although these assignments have been chosen as a fit for the experimental data, the water cluster model is established [55] and fits the closely related hydrated polyoxomolybdate nanocapsule as examined by X-ray diffraction [547]. [Back to Top ![]() ]
]
The 1165 polyoxomolybdate {Mo154} nanowheels distributed on the surface of the 90 nm spherical cluster [1180] must be separated from each other by several hydration layers (on average) as the center-to-center distance between two adjacent nanowheels would be about 5.0 nm using hexagonal, or 4.7 nm using square, close-packing whereas the surface-surface diameter of an individual nanowheel is only 3.8 nm [1180]. Even if a surface could be formed from the nanowheels utilizing just three neighbors for each nanowheel (for example, not close-packed but with similar topology to the fullerenes, see later), these would have to be 4.1 nm apart at their extremities, still necessitating the utilization of interfacial water molecules. Additionally, such a structure based on three-fold symmetry would not fit with their seven-fold symmetry. It has been suggested that the driving force for the assembly of these structures is a delicate balance between attractive short-range forces (van der Waals forces and hydrogen-bonding forces) and repulsive electrostatic interactions between adjacent anion clusters [1180b]. Due to the extended time required for forming these clusters and the changes in dielectric spectra and small-angle X-ray scattering (SAXS) [1180a] on solution aging, it is clear that the clustering must take place in stages.
There are four experimental datasets that the hydration and clustering of the {Mo154} nanowheels have to conform; dielectric spectroscopy [1181], small-angle X-ray diffraction [1180a], light scattering [1180b], and scanning electron microscopy [1180]. In addition, the proposal cluster growth must be soundly based.
The conductance-corrected dielectric difference spectra of the aged 1.82 mM solution, on nanowheel vesicle formation, are shown below right (blue line) [1181] and compared with that of the fresh solution (dashed red line) discussed above.
Dielectric difference spectra of aa aged 1.82 mM {Mo154}
nanowheel solution (blue);
fresh solution is shown red dashed, from [1181]
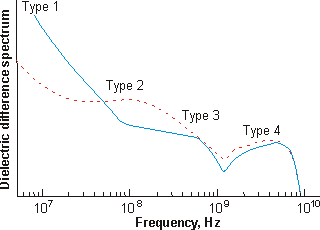
| Water |
Relaxation time |
Amplitude change |
|---|---|---|
| Type 1 | Stays at ≈ 22 ns | Almost doubles |
| Type 2 | Stays at ≈ 1.7 ns | Reduces by more than 50% |
| Type 3 | Increases from 0.2 ns to 0.35 ns | Reduces to 60% |
| Type 4 | Stays at ≈ 0.04 ns | Stays about the same |
| Bulk | Stays at ≈ 0.00822 ns | Stays about the same |
The conclusions that can be drawn from this dielectric data is that more type 1 water is strongly bound, less type 2 water is found and is bound less-strongly, less type 3 water is found in the cavities but it is more strongly bound, and the type 4 surface-bound water is unaffected as is the bulk water.
The distribution function for a fresh solution (blue dotted) and
from the same solution after two days(red line), from [1180]
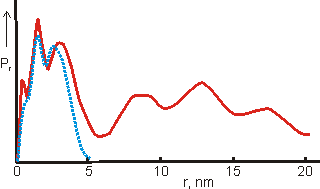
Shown opposite is the distance distribution function from small-angle X-ray scattering (SAXS) data obtained from a freshly prepared solution of the {Mo154} nanowheels (blue dotted) and from the same solution after two days (red line) [1180], showing the formation of oligomers.
The first stage in the vesicle formation from {Mo154} nanowheels must be the formation of dimers utilizing a similar water clustering arrangement to that proposed above for monomer hydration and found in supercooled water (ES) and the hydrated polyoxomolybdate nanocapsule [547].
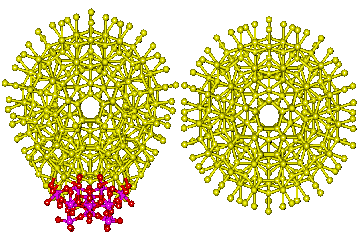
The 14 possible linking surfaces] of the nanowheel
![The 14 possible linking surfaces] of the nanowheel The 14 possible linking surfaces] of the nanowheel](images/giantl.gif)
The cartoon right shows the putative hydration linking faces on the {Mo154} nanowheel based on the 14 [(Mo)Mo5Mo5O33.(H2O)5] anchor points.
Hydrating water is colored yellow with the linking faces colored blue (only the O-atoms of water are shown). Every hydrated {Mo154} nanowheel has 14 possible linking surfaces based upon the pentagonal anchors shown above. In this cartoon, all icosahedral clusters have been truncated at their putative linking surfaces. In the hydrated oligomers, unused linking surfaces would be expected to retain their icosahedral clustering, with somewhat enhanced hydrogen bonding by the more extensive oxomolybdate surfaces. During the formation of the 90 nm spherical supercluster, described below, hydrated {Mo154} nanowheels develop from tetramers. In each tetramer, two nanowheels are linked by their 1, 6, and 10 faces, one by its 1, 4, and 7 faces and the fourth by its 1, 9, and 12 faces. An extra linkage to either, but not both, these latter two nanowheels may be formed through their 11 or 5 faces, respectively.
The water network linking two anchor points
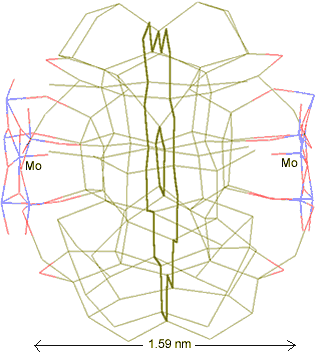
Thus, two {Mo154} nanowheels may link through a water network involving two pentagonal boxes (one associated with each [(Mo)Mo5Mo5O33.(H2O)5] anchor point), with the topology shown left. The water O-atoms are colored green, with the H-atoms left out for clarity. The central pentagon Mo---Mo distance between clusters is about 1.59 nm.
The complete hydrated dimers with their complete icosahedral clusters on the unlinked surfaces are presented below. The interactive structure of this cluster is available (Jmol).
Hydrated dimers surrounded by their icosahedral clusters
Two dimers form a tetramer {Mo154}4
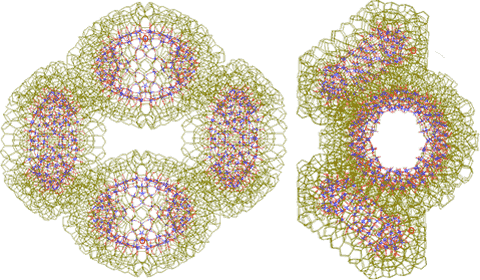
Two {Mo154}2 dimers can form a tetramer {Mo154}4, stabilized by four of the hydrogen-bonded linking water clusters.
Such tetramers have been previously suggested as the dominating cluster from SAXS studies [1180].
The interactive structure of this cluster is available (Jmol)
A tetramer adds a nanowheel to form a pentamer
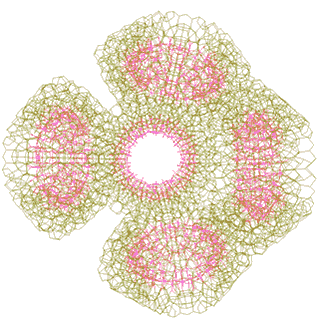
Each tetramer can add one further {Mo154} nanowheel to one of two alternate positions to form a pentamer (these positions are shown by the red circles in the figure above, and the complete pentamer is shown left) as indicated above. This additional {Mo154} nanowheel may be added at any stage in the further clustering towards the nanoshell formation. However, space exists for no more than one {Mo154} nanowheel to be can be added to close the structure of each tetramer, so being stabilized by neighboring icosahedral water clustering.
Two tetramers link to form an octamer ({Mo154}4)2
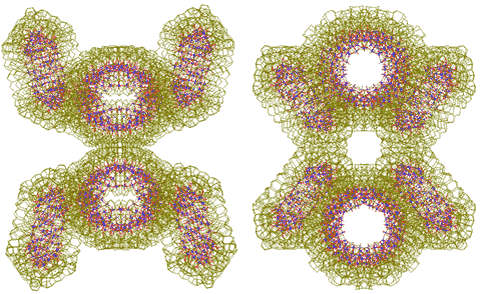
Two tetramers (or pentamers) can further join through two linking water clusters to form octamers ({Mo154}4)2 (or decamers). See right for nanowheel octamers, as viewed from different directions.
Three of the octamers shown above can cluster into a distorted [({Mo154}4)2]3 hexagon
![Three of the octamers shown above can cluster into a distorted [({Mo154}4)2]3 hexagon]](images/gianthex.gif)
Three of the octamers shown above can cluster into a distorted [({Mo154}4)2]3 hexagon as shown left. These distorted hexagons thus contain 24 {Mo154} nanowheels held together by 33 linking water clusters. As shown later, these octamers may then form the surface of the nanoshell supercluster.
The distance between tetrameric clusters (12.36 nm) and the diameter of the distorted hexagon (mouse image to view) have been found close to the major peak in the SAXS experimental data (12.7 nm) [1180].
Five {Mo154}4 tetramers may also cluster to form a regular pentagon
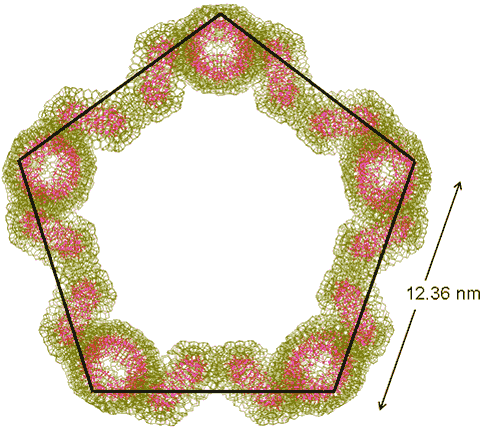
Five {Mo154}4tetramers may also cluster to form a regular pentagon (with 20 {Mo154} clusters, shown on the right) and six {Mo154}4 tetramers may cluster to form a regular hexagon ring (with 24 {Mo154} clusters). Such rings are formed from singly-linked tetramers but stabilized by the ring closure. They are also formed consequent upon the clustering of the distorted hexagons (above).
It is proposed that the final assembly of the nanosphere is made up of distorted hexagons in such a way that undistorted pentagons and hexagons are also formed (see below).
The nanosphere is made up from the distorted hexagons in such a way
that undistorted pentagons and hexagons are also formed
Animated gif of rotating nanoshell
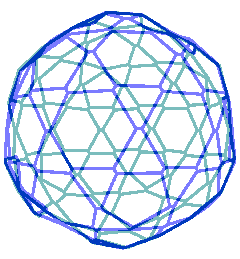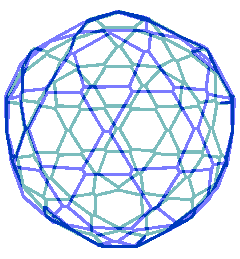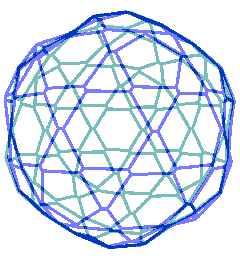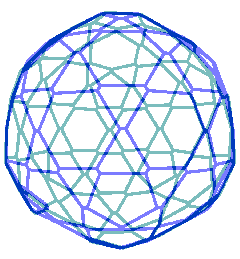
Each vertex in this nanoshell is the Mo atom at the water hydrogen-bonded linkage of a tetramer. The 90 nm nanosphere has 240 vertices requiring a minimum of 960 {Mo154} nanowheels. Without altering the shell diameter, each of the 240 {Mo154}4 tetramers may add a further {Mo154} nanowheel to give a maximum content of 1200 {Mo154} nanowheels if completely assembled from {Mo154}5 pentamers.
The interactive structure of this cluster is available (Jmol).
Shows the spherical nanoshell has the same topology as a fullerene, C240, on left
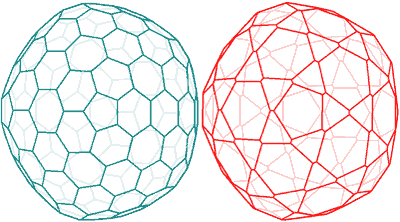
The spherical nanoshell has the same topology as a fullerene, C240. The C240 is only 1.44 nm in diameter, whereas the 'model' {Mo154} has a surface-surface diameter of ≈ 90.6 nm. This may be compared with the diameter determined experimentally by light scattering from the radius of gyration of 90.4±2.8 nm.
The connectivity map for the proposed spherical nanoshell
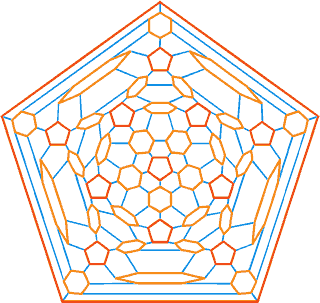
The diagram opposite is the connectivity map (Schlegel diagram) for the proposed spherical nanoshell. The 12 pentamers (red), 30 symmetric hexamers (orange) and 80 distorted hexamers that make up the spherical shell are easily seen. The shells can either be thought of as formed from 12 regular pentagons (that is, 12 ˣ 20≈ 24 {Mo154}) plus 30 regular hexagons (that is, 30 ˣ 24≈ 30 {Mo154}) linked together, or as formed from 80 distorted hexagons with shared edges (that is, 80/2 ˣ 24≈ 30 {Mo154}).
The route to the supercluster involves a sequence of associations with an increasing proportion of linkages providing the enthalpic gain to overcome the decreasing entropy of formation [1183]:
{Mo154} + {Mo154} ![]() {Mo154}2
{Mo154}2
2 {Mo154}2 ![]() {Mo154}4
{Mo154}4
2 {Mo154}4 ![]() ({Mo154}4)2
({Mo154}4)2
3 ({Mo154}4)2 ![]() [({Mo154}4)2]3
[({Mo154}4)2]3
40 [({Mo154}4)2]3 ![]() ([({Mo154}4)2]3)40
([({Mo154}4)2]3)40
240{Mo154}+([({Mo154}4)2]3)40 ![]()
![]()
![]() ([({Mo154}5)2]3)40
([({Mo154}5)2]3)40
Links/{Mo154}
0.5
1.0
1.25
1.375
1.5
1.4
In this way, the spherical supercluster ([({Mo154}4≈ 5)2]3)40 is built up with an increasing number of links per {Mo154} nanowheel used, which overcomes the reduced entropy. The final {Mo154} content is 960-1200, which agrees with the average of 1165 that has been found experimentally. Once the 90 nm spherical superclusters are formed, they will retain weak linkage positions on the outside that can enable their aggregation, as seen in the scanning electron microscope images [1180]. [Back to Top ![]() ]
]
a It is not thought simply to be a nanobubble (nanocavity, for example, see [1172]), as the {Mo154} nanowheel is not amphiphilic, the size of the nanosphere is exact, and the pressure inside may otherwise be unstably high at 3.2 MPa if the Laplace equation holds. If nanobubbles (nanocavities) were involved, it would be expected that the amount of nanoparticles involved would be independent of the size of the nanobubbles (as the nanoparticles congregate favorably at the gas/liquid interface), which is not the case here. Also, there would be expected to be a need for an amphiphilic solute [1172]. [Back]
b A useful and interesting review of this area has been written, which presents a different hypothesis to the one described here [1223]. [Back]
Home | Site Index | Interactive structures (Jmol) | Polyoxomolybdate nanodrop | LSBU | Top
This page was established in 2007 and last updated by Martin Chaplin on 24 October, 2021