
Gelatin dessert

Gelatin is a gelling agent forming transparent elastic thermoreversible gels.
Gelatin (also called gelatine) is is an animal protein prepared by the thermal denaturation of collagen (for the structure of collagen, see [2730]), isolated from animal skin and bones, with very dilute acid. It can also be extracted from fish skins.
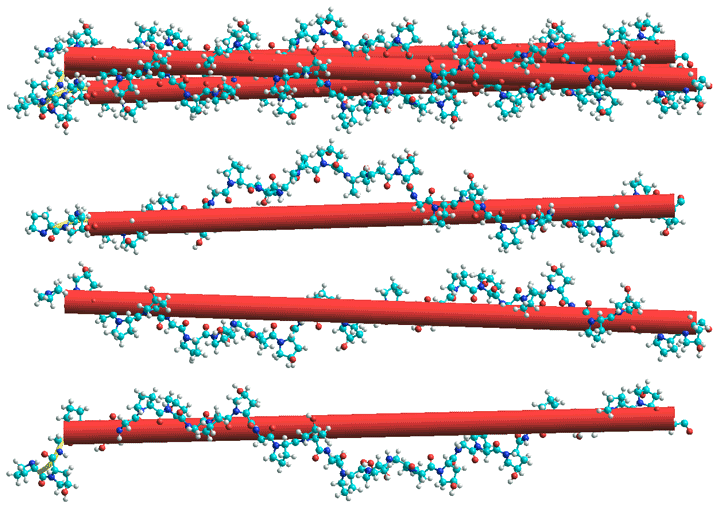
Collagen, triple helix shown top with the three helices separated below.
Intermolecular hydrogen bonds stabilize the fibrils.
from Protein Data Bank (PDB) entry 1cag
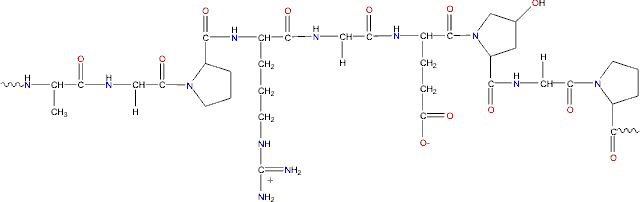
Gelatin contains many glycine residues (almost 1 in 3 residues, arranged every third residue), proline, and 4-hydroxyproline residues. A typical structure is -Ala-Gly-Pro-Arg-Gly-Glu-4Hyp-Gly-Pro-.
Representative gelatin structure; Ala-Gly-Pro-Arg-Gly-Glu-4Hyp-Gly-Pro
[Back to Top ![]() ]
]
Gelatin is a heterogeneous mixture of single or multi-stranded polypeptides, each with extended left-handed proline helix conformations and containing between 50 - 1000 amino acids. The triple helix of type I collagen extracted from skin and bones, as a source for gelatin, comprises two α1(I) and one α2(I) chains, each with molecular mass ≈ 95 kD, width ≈ 1.5 nm, and length ≈ 0.3 μm. The hydration of a collagen-like protein has been described [2868]. Water molecules hydrogen-bond to the hydroxyl groups of hydroxyproline and the carbonyl groups of the peptide forming both intra-chain and inter-chain links. Thus, the triple helices are coated by a cylinder of hydration with their grooves filled with solvent molecules, and this coating maintains the collagen's conformation and mechanical properties. Dielectric relaxation studies of collagen have shown that the water associated with collagen is highly ordered with long-range perturbations beyond the first hydration shell [3350].
Gelatin consists of mixtures of these strands together with their oligomers and breakdown (and other) polypeptides. Solutions undergo coil-helix transition, followed by aggregation of the helices by forming junction zones of collagen-like right-handed triple-helices. Higher levels of pyrrolidines result in stronger gels. There is some dispute over whether each of the three chains in the helical structure has a 10/1 helix (the three strands forming a 10/3 helix) with an 85.8 Å axial repeat or a 7/1 helix (the three strands forming a 7/2 helix) with a 60 Å axial repeat, with tripeptides forming each unit. Although the former view seems prevalent, further evidence indicates the latter to be correct [1054]. Each of the three strands in the triple helix requires about 21 residues to complete one turn; typically, there would be between one and two turns per junction zone [449]. Gelatin films containing greater triple-helix content swell less in water and are consequentially much stronger [632]. Chemical cross-links can be introduced to alter the gel properties, using transglutaminase to connect lysine to glutamine residues [246] or glutaraldehyde to connect lysine to lysine.
There are two types of gelatin dependent on whether or not the
preparation involves an alkaline pretreatment, which converts asparagine
and glutamine residues to their respective acids and results in
higher viscosity. Acid pretreatment (Type A gelatin) uses pigskin,
whereas alkaline treatment (Type B gelatin) uses cattle
hides and bones. [Back to Top ![]() ]
]
Gelatin is primarily used as a gelling agent [319], forming transparent elastic thermoreversible gels on cooling below about 35 °C, which dissolves at low temperature to give 'melt in the mouth' products with useful flavor-release. Also, the amphiphilic nature of the molecules endows them with useful emulsification (for example, whipped cream) and foam-stabilizing properties (for example, mallow foam). On dehydration, irreversible conformational changes occur [397] that may be used to form surface films. Such films are strongest when they contain greater triple-helix content. Gelatin is also used as a fining agent to clarify wine and fruit juice. In 2021, gelatin was developed into non-melting ice cubes to cool foodstuffs [4396]. These are made of about 90% water and may be shaped, but are recyclable, biodegradablie. and importantly may be used in repeated freeze–thaw cycles.
Although gelatin is by far the major hydrocolloid used for gelling in the food industry, current concerns about the possibility of such an animal-derived product, possibly containing the prions that cause Creutzfeldt-Jakob Disease (CJD)a. Also, the need generated by vegetarians and certain religions, has encouraged the serious search for gelatin alternatives. However, the combination of the melt in the mouth, elastic, and emulsification characteristics of gelatin gels is difficult to reproduce.
Gelatin is nutritionally lacking as a protein being deficient in isoleucine, methionine, threonine, and tryptophan.
Interactive structures are available (Jmol). [Back to Top ![]() ]
]
a It should be noted that gelatin is prepared under harsh conditions that make it effectively impossible for the survival of the CJD-causative prion, even in the unlikely circumstance of it being present. However, concern exists due to the severity of the disease, the known stability of the prion taken, and the difficulty of analyzing for it at the extremely low levels that may cause the disease. [Back]
Home | Site Index | Hydrocolloids | Polysaccharide hydration | hydrogen-bonding | LSBU | Top
This page was established in 2002 and last updated by Martin Chaplin on 3 January, 2022