
Part of a polysaccharide
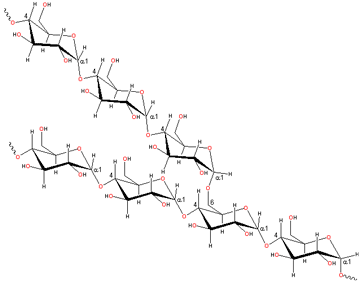
Polysaccharides are polymers containing many hydroxyl groups and generally interact strongly with water.
![]() Introduction
to polysaccharides
Introduction
to polysaccharides
![]() Hydration
Hydration
![]() Alternatives for defining bound and unbound water
Alternatives for defining bound and unbound water
![]() Polar effects, for example, α-D-galacturonic
acid
Polar effects, for example, α-D-galacturonic
acid
![]() Weak hydrogen-bonding, for example, α-L-arabinofuranose
Weak hydrogen-bonding, for example, α-L-arabinofuranose
![]() Strong hydrogen-bonding, for example, β-1-4-linked
D-xylose
Strong hydrogen-bonding, for example, β-1-4-linked
D-xylose
![]() Hydrophobic effects, for example, β-1-4-linked
D-xylose
Hydrophobic effects, for example, β-1-4-linked
D-xylose
![]() Effects of other solutes: non-ionic
Effects of other solutes: non-ionic
![]() Effects of other solutes: ionic
Effects of other solutes: ionic
![]() Conclusions concerning polysaccharide hydration
Conclusions concerning polysaccharide hydration
Using a simplistic approach to polysaccharide hydration, water can be divided into 'bound water', c subcategorized as being capable of freezing or not, and 'unbound water', subcategorized as being trapped or not, and with 'hydration' as a general term concerning the amount of bound water. The term 'bound' is poorly understood, being very difficult to explain (or investigate) exactly, and has been defined (perhaps in desperation) as 'non-bulk' water. 'Unbound' water freezes at the same temperature as normal water (< 0 °C dependent on cooling rate). However, some water may take up to 24 hr to freeze. 'Bound freezable' water freezes at a lower temperature than normal water, being easily supercooled. It also exhibits a reduced enthalpy of fusion (melting). Although the inability to freeze is often used to determine bound water, freezing may not a good measure of hydration as it concerns the water content of the glassy state and not aqueous hydration. However, alternative determinants of 'bound' water, such as the use of NMR, are also problematic as NMR determines binding dynamics (rates of dissociation involving dissociation activation energy) rather than thermodynamics (free energy changes). Reaction of polysaccharides to form unnatural analogs, such as through methylation, carboxymethylation, or deoxyfluorination, usually cause great changes in their mechanical and structural properties and allow the establishment of detailed structure-property correlations [3814].
A high degree of branching in a polysaccharide (or crosslinking) has been found to lead to a more low-density water structuring (ES-like ) and stronger binding of the water micro-pools [2643]. [Back to Top ![]() ]
]
For alternative (1), the type of water is determined by
(a) freezing and melting calorimetry using the enthalpy of the phase change [1514, 3050],
(b) thermogravimetry, measuring the weight changes due to absorption from set humidity atmospheres, and
(c) 1H NMR spin-lattice (longitudinal, T1 ) and spin-spin (transverse, T2 ) relaxation times [3050].
The presence of sufficient freezing bound waterb is required for preferred biocompatibility. It is supposed that such a layer shields the inner non-freezing water layer from the hydration shell of other biological components [1700]. Non-freezing water may be trapped in a glassy state, lowering diffusion by several orders of magnitude and hindering crystal formation.
For the less precise alternative (2), which is used in the dietary fiber area, the firmly held water not removed by centrifugation, gives the water binding capacity (WBC), whereas the loosely associated water, which is not removed by filtration, gives the water holding capacity (WHC).
However, both schemes only determine what they measure which may be somewhat different from what they represent as measuring; that is aqueous hydration. At present, the literature provides little sound explanatory theory for polysaccharide hydration, with poor predictability. Another variable concerns dried polysaccharides; the method of drying leaves different amounts and forms of the remaining water with some structural properties unchanged (Infrared Spectroscopy and X-ray Diffraction spectrum) while others are changed (Low Field Nuclear Magnetic Resonance) [3900]. Vacuum freeze-drying, vacuum drying, and hot-air drying methods have given differences in scavenging free radicals tests.
In practical experience, the effects of water on polysaccharides and polysaccharides on the water are complex and become even more complex in the presence of other materials, such as salts. Water competes for hydrogen-bonding sites with intramolecular and intermolecular hydrogen-bonding, which certainly will determine the carbohydrate's flexibility and may determine the carbohydrate's preferred conformation(s) [254]. There is a high entropic cost (up to about 20.8 kJ ˣ mol−1 at 25 °C for a totally 'frozen' molecule) when water is bound, and this must be reclaimed, for example, by the formation of stronger or extra hydrogen bonds [320]. There are several questions to be answered:
These questions can be answered by considering the following effects on the polysaccharide hydration: polar, weak hydrogen-bonding, strong hydrogen-bonding, hydrophobic, and the presence of other non-ionic and ionic solutes. a
[Back to Top ![]() ]
]
α-D-galacturonic acid
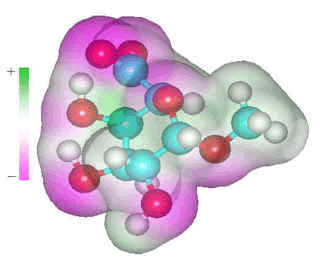
D-galacturonic acid residues occur in pectins and tightly bind some water molecules. However, the amount bound depends mainly on the cation counter-ion present rather than the sugar residue. Thus, sodium galacturonate binds 4 molecules, and the K+, Ca2+ and Mg2+ salts bind 2, 12, and 13 water molecules respectively (water-ion binding greater than 13.0 kcal mol−1 [250]). Both acid oxygen atoms of the carboxylate group prefer to accept two or three hydrogen bonds, dependent on steric factors, each provided from different water molecules due to steric and anti-cooperative effects, and as shown by an ab initio study [316].
As the distance between the two carboxylate oxygen atoms (O6A····O6B) is smaller (at about 2.2 Å) than H2O····HOH hydrogen-bonded O····O distance (at about 2.8 Å), these water molecules will only fit in a locally collapsed, weaker, water structure.
As hydrogen-bonding (through donation) is weakened if one of the donor hydrogen bonds of water is hydrogen-bonded to a stronger base than water, carboxylates are expected to give rise to a particularly weak hydrogen bond in the next shell, so encouraging a local collapse in the hydrogen-bonded network. Near polyelectrolytes, the osmolality is high, and water activity and chemical potential are low. The potential of water is partially increased by collapsing the hydrogen bond network. If the surface is highly charged, the high-density water (HDW) zone may reach out to several nanometers, and the local density of the first hydration shell may be greater than 1.1 g cm−3. The zone is weakly hydrogen-bonded, fluid, and reactive, and accumulates small cations, multivalent anions, and hydrophobic solutes. In order to keep the potential of the water constant, the water surrounding this low potential HDW zone is reduced in potential to match, producing a zone of lower density water (LDW), which may be extensive enough as to lower the overall density of the medium [407]. These two zones (HDW) and (LDW) are unlikely to be sharply distinguishable or perfectly formed, but the chemical potential of the water will be similar throughout. This may be the case, as found in the domain structure of solutions [1148].
The polysaccharide chain rigidity will depend on whether the counter ions are close (leading to possible intermolecular attraction but reducing the intramolecular charge repulsion) or far away (for example, in the LDW). The rules for the formation of ion-pairs are followed with the carboxylate group considered to be poorly hydrated, so strongly hydrated ions (for example, Li+, Ca2+, Mg2+) will adsorb to the ionic surfaces less readily than poorly hydrated ions (for example, Cs+, K+, Na+).
Some poly-ionic polysaccharides (for example, pectins with more than 57% unmethylated carboxylate groups) can form junction zones utilizing divalent cations (for example, Ca2+) so long as a minimum of 14 residues can cooperate. It may well be that two carboxylate groups have to cooperate in prizing the bound water away from the calcium ions to form the salt links that make up the junction zones. The low potential of the water associated with the ion pairs will affect nearby water to encourage a leveling of the water potentials. This causes surrounding water to become more expanded (lower in density).
The ζ-potential of polysaccharides is frequently used to understand polysaccharide-protein complexation, but the actual binding depends on how close the protein can get to the charged sites [3136].
[Back to Top ![]() ]
]
α-L-arabinofuranose
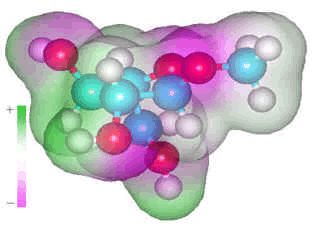
α-L-arabinofuranose occurs in arabinoxylans and cannot form intra-residue hydrogen-bonding due to the position of its alcohol groups and the fluid nature of the furanose ring which is continually changing its conformation due to the low potential energy barriers between a large number of conformers with similar potential energies. However, it does form many single hydrogen bonds to water molecules. This hydrogen bonding is weak and destructuring, with every carbohydrate hydroxyl group acting as a donor and preferably also as a double acceptor.
As well as these three water molecules, there is likely also to
be 1-2 non-bonded nearest neighbors. Similarly, ring oxygen atoms
will have 1-2 water molecules associated with them, plus 0-1 non-bonded
nearest neighbors, and glycosidic oxygen atoms have one water molecule
associated with them, plus 0-1 non-bonded nearest neighbor. [Back to Top ![]() ]
]
β-1-4-linked D-xylose
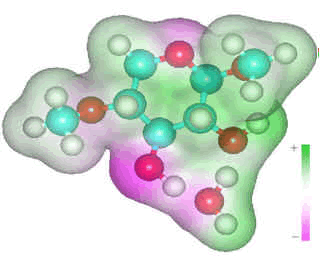
Strong hydrogen bonding requires
two hydrogen bonds from the polysaccharide to the same water
molecule, one as a donor and one as an acceptor for maximum cooperativity.
Strong (double) hydrogen-bonded water links often appear to
displace single intra-residue hydrogen bonds as they have
similar requirements for suitably oriented vicinal hydroxyls,
and their presence can reduce the stereochemical demands.
Such hydrogen bonding is likely to be stronger if the hydroxyl
groups have less flexibility. Thus, hydrogen-bonding strength
will generally follow the trend: neighboring on pyran (for example, as shown opposite) > neighboring on furan > between
hydroxyl groups on neighboring residues > between ring
hydroxyl and methylhydroxyl groups. Polysaccharides are more hydrophobic
if they have intra-molecular hydrogen bonds. However, the fixed orientation of the doubly-linked
water molecules may increase the hydrophobic extent of the carbohydrate's
top and bottom surfaces and so aid the formation of junction zones. This is a cooperative process that may take some
time. If the doubly bound water is replaced by two singly bound
water molecules, the polysaccharide will become less hydrophobic,
there will be a further entry of water and a partial break-up of
any junction zones. However, as the formation of junction zones
is a cooperative process, it will be difficult to reverse. Also,
singly-linked water molecules involve greater (energetically unfavorable)
rearrangements in the surrounding water. If all other factors are
equal, the preferences being: intramolecular hydrogen-bond >
doubly hydrogen-bond linked water > two singly hydrogen-bond
linked water molecules. [Back to Top ![]() ]
]
β-1-4-linked D-xylose
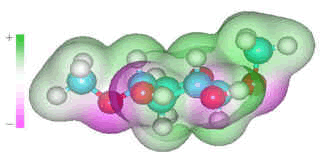
Several carbohydrates (for example, β-1-4-linked-D-xylose in arabinoxylans, β-1-4-linked-D-glucose in cellulose and β-glucans, β-1-3-linked-D-glucose in β-glucans and β-1-3-linked-D-xylose) may be considered as having two hydrophobic surfaces with a hydrogen-bonding edge. The "hydrophobic effect" is primarily a consequence of changes in the clustering in the surrounding water rather than water-solute interactions.
Where low-density water (LDW) overlays hydrophobic surfaces, there
will be a tendency for surface minimization by surfaces interacting
and excluding water, causing the formation of junction zones. It
is the incompatibility between the LDW and the hydrophobic surface that drives the structure formation.
The formation of low-density water next to hydrophobic surfaces
and concomitant junction zone formation are encouraged if this low-density
water is also associated with high-density water created near
polar groups (see above). Alternatively, strong local
hydrogen-bonding may be able to create low-density water
without assistance. It is likely, however that local weak hydrogen
bonding will discourage junction zone formation. It is noteworthy
that the hydrophobic effect decreases with increased pressure (or
density) as it is dependent on the presence of tetrahedrally-placed
water molecules (as in LDW), which reduce in number under the distorting
influence of pressure [626]. [Back to Top ![]() ]
]
Kosmotropes are very soluble, well-hydrated molecules, having no net charge and enforcing extensive hydrogen bonding. They may 'compensate' for the disrupting effects of high ionic concentrations in some natural microorganisms. Kosmotropes are molecules that stabilize the structure of macromolecules in solution. They stabilize polysaccharide junction zone formation in the same way as they are preferentially excluded from their surfaces. This exclusion entropically 'drives' junction formation. Low molecular weight sugars can cause hydrogen-bonding links between polysaccharides by dehydrating the surface.
The effects of dissolved gases are often ignored. However,
they are usually present (even in distilled and de-ionized
water), and may have important and varying effects [711].
Some gases are essentially structuring (for example, O2,
N2, Ar), whereas others are destructuring (for example, CO2). Structuring gases,
even with their low solubility, may accumulate at hydrophobic
surfaces (including the formation of tiny gas bubbles [459]
that may grow, if sufficient gas is available in solution,
to form gas-filled capillaries [578])
where they are more soluble (the water here possessing effectively
very low relative permittivity (dielectric constant)) and so increase their structuring
effects. Such dissolved gas has a major effect on emulsion
stabilization and flocculation (degassing stabilizing the
emulsions by removing the (strange) long-range hydrophobic
attraction [711]) and confusing
any Hofmeister effects [671].
They may also be responsible for other strange effects such
as free radical production in electromagnetic
fields. [Back to Top ![]() ]
]
Ions generally have an effect on the structure of water like increased
temperature or pressure. Small ions are strongly hydrated, creating
local order but destroying the natural hydrogen-bonded network.
The Hofmeister series shows the promotion
of hydrophobic associations, such that ions favoring low-density
water promote amylose retrogradation and starch gelatinization.
Ions in polysaccharide solutions may behave differently from when
in solution by themselves, as the polysaccharides are capable of
producing relatively stable low-density or high-density aqueous
microenvironments. If LDW accumulates solute at a charged interface, it will relax back to average density, as will the associated HDW. Structure-making salts concentrate in a less structured
aqueous phase and decrease the compatibility between hydrophilic
polymers, while structure-breaking salts concentrate in more structured
environments and increase such compatibility. [Back to Top ![]() ]
]
Formation of junction zones leads to compartments where the clustering of water reduces efflux, with clusters of 20-30 molecules having difficulty getting through the pores.
[Back to Top ![]() ]
]
a A modeling study has characterized the hydration around a number of mannose and glucose oligosaccharides [1600]. [Back]
b Non-freezing water occurs below the glass transition point. Therefore, the question arises over whether the hydrogen-bonding or the glass transition causes the 'non-freezing' as both causes have been claimed. In this case, it is a cause and effect phenomenon, with hydrogen-bonding being the cause and the glass transition being the effect. Of course, glass transitions can occur with dry materials without water (as in silica glasses), but the author considers the hydrogen bonds necessary in this case. A case has been made to disprove this hypothesis whereby polysaccharide glass transitions are almost independent of hydrogen-bonding [2594]. [Back]
c Many possible definitions of 'bound' have been used in the literature, depending on the circumstances. The broadest definition is that 'bound' water is that water in the vicinity of a macromolecule whose properties differ detectably from those of the 'bulk' water in the same system. 'Free' water is that water not 'bound'. This definition depends on the detection system used, with other definitions of 'bound' lying with boundaries within this definition's boundaries. Often there is no sharp physical 'boundary' that separates 'bound' from 'free' or' bulk' water. [Back]
Home | Home | Site Index | Hydrocolloids | Protein hydration | Nucleic acid hydration | Aqueous biphasic systems | LSBU | Top
This page was established in 2001 and last updated by Martin Chaplin on 25 October, 2021