
α-D-glucose
Sugars are very soluble in water, forming many hydrogen bonds.
Sugar hydration depends upon the balance between intra-molecular hydrogen-bonding and hydrogen-bonding to water. This balance involves several layers of hydrating water molecules. A modeling study has characterized the hydration around several saccharides [1600].
Sugar hydration, exemplified by inositol hydration
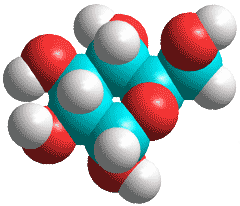
Some sugar molecules can fit into a network structure of icosahedral water clusters, with hydrogen-bonding, by replacing a chair-form water hexamer in a cluster. Equatorial alcoholic oxygen atoms may be placed in similar positions to the oxygen atoms of the water molecules. Such a strengthened network around the sugars contributes to forming a stable sugar-water glassy state and the obstruction of the crystallization process on cooling.
Thus, in the case of scyllo-inositol a (shown left), which has six such oxygen atoms, each hydroxyl group can both donate and accept a hydrogen bond, where the remaining water molecules have been removed to clarify the image.
Note that the orientations of all the hydroxyl groups are different from those in the gas phase molecule shown below.
Inositol structure modeled in a vacuum
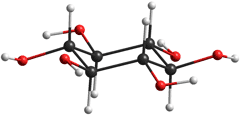
The counterclockwise equatorial arrangement of hydroxyl groups (right) is probably down to the oxygen lone pair repulsion rather than the very weak hydrogen bonds [2148]. Hydration forces easily overcome such weak interactions.
More detailed interactive structures are available (Jmol). These show how the scyllo-inositol fits into the complete network of hydrogen bonding within icosahedral water clusters.
Although scyllo-inositol is not the most stable inositol in the gas phase (using ab initio calculations, relative to myo-inositol and neo- inositol bearing one and two axial hydroxyl groups, respectively), it becomes the most stable inositol isomer when dissolved in water [2443]. 17O Spin-lattice relaxation times have confirmed
inositol's strongest interaction with water compared
with a range of monosaccharides and their reduced polyols
[307]. Such interactions
ensure that no hydrogen bonds are left 'dangling' in the water
cluster. As the chair-form water
hexamers are the least affected part of the icosahedral
water clusters by the ES ![]() CS equilibrium structural changes,
sugars may stabilize preferentially either the ES or CS hydrogen-bonded network, dependent on their conformation.
Equatorial hydroxyl groups on chair-form sugars show stronger
interactions [307] as the water
molecules are optimally positioned for more extensive and
stronger hydrogen bonding. This explains why
the beta anomers of D-xylopyranose and D-glucopyranose predominate
in solution in contrast to ab initio modeling predictions
[324]. The tendency
for the scyllo-inositol to stretch the water structure towards ES is at an energetic cost (particularly of the entropy
of hydration), however, and this is minimized by reducing
its solubility. b A similar
effect can be seen in the low solubility of β-glucans, β-xylans,, and β-mannans,
where the fit of their 1-2, 1-3, 1-4, 2-3, 2-4, and 3-4 O-O
distances with that of ES water neighbors (2.82 Å, 4.60 Å, 5.40 Å, 2.82 Å, 4.60 Å, 2.82 Å, respectively)
can be seen below (mean square deviations).c
CS equilibrium structural changes,
sugars may stabilize preferentially either the ES or CS hydrogen-bonded network, dependent on their conformation.
Equatorial hydroxyl groups on chair-form sugars show stronger
interactions [307] as the water
molecules are optimally positioned for more extensive and
stronger hydrogen bonding. This explains why
the beta anomers of D-xylopyranose and D-glucopyranose predominate
in solution in contrast to ab initio modeling predictions
[324]. The tendency
for the scyllo-inositol to stretch the water structure towards ES is at an energetic cost (particularly of the entropy
of hydration), however, and this is minimized by reducing
its solubility. b A similar
effect can be seen in the low solubility of β-glucans, β-xylans,, and β-mannans,
where the fit of their 1-2, 1-3, 1-4, 2-3, 2-4, and 3-4 O-O
distances with that of ES water neighbors (2.82 Å, 4.60 Å, 5.40 Å, 2.82 Å, 4.60 Å, 2.82 Å, respectively)
can be seen below (mean square deviations).c
| β-D-Glc | α-D-Glc | β-D-Man | α-D-Man | β-D-Gal | α-D-Gal | β-D-Xyl | α-D-Xyl |
|---|---|---|---|---|---|---|---|
| 0.02 | 0.16 | 0.03 | 0.29 | 0.18 | 0.17 | 0.02 | 0.17 |
Comparing the sugars with hexagonal ice structure and allowing for the natural anomeric ratios using an atomistic Monte Carlo simulation gives a different order: D-galactose> D-glucose> D-mannose [2282].
Cooperative intramolecular hydrogen-bonding in carbohydrates depends on the equatorial/axial orientation of the hydroxyl groups. Such intramolecular hydrogen-bonding reduces the hydration of the carbohydrates, increases their nonpolar character, and is key to their biological recognition [981]. Simulations of β-D-glucose, β-D-mannose, β-D-galactose, and β-D-talose using explicit water molecular dynamics gave generally acceptable structural, dynamical, solvation, and energetic agreement with the available experimental data [1216]. First-principles molecular dynamics of glucose in water has shown that each hydroxyl group forms about two hydrogen bonds; forming a weaker acceptor and a stronger donor to water which together fit relatively poorly (if compared with scyllo-inositol) into a locally tetrahedral network [1367]. Using NMR, the hydroxy protons of polyols in aqueous solutions give information concerning their water exchange and hydrogen bonding strengths [1216].
In contrast to internal hydroxyl groups (secondary and tertiary -OH groups), terminal hydroxy protons (primary -OH groups) are in a fast chemical exchange with water molecules and do not show hydrogen bonding using NMR [3504]. The innermost hydroxy protons (those surrounded by secondary or tertiary -OH groups) form even stronger hydrogen bonds. Reducing sugars undergo mutarotation in solution forming an equilibrium mixture of the α- and β-forms. This mutarotation process is catalyzed by water molecules [1009]. Although the equilibrium position is reportedly determined partially by the water clustering, there appear to be no differences in molal volume or specific heats between the anomers [1421]. The structure of reduced carbohydrates (polyols) seems more compatible with water when they form planar zigzag structures, such as mannitol but not sorbitol, and where their alcohol groups fit in well with water's second neighbor oxygen distances [1421].
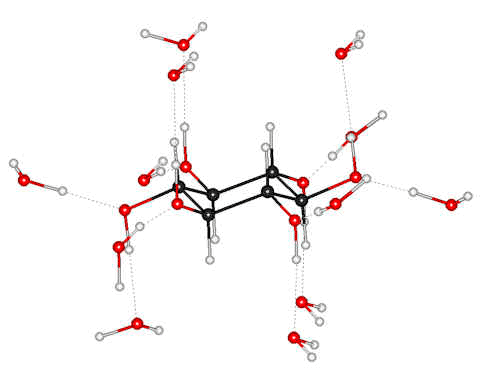
β-D-glucose hydrated with 15 H2O, [4056]
![ß-D-glucose hydrated with 15 H2O, [4056] ß-D-glucose hydrated with 15 H2O, [4056]](images/gluc15.gif)
Using terahertz (THz) spectroscopy, glucose appears to have 21 bound d water molecules, higher than expected, plus other affected water molecules, both hydrogen-bonded and not hydrogen-bonded to these [2379]. Due to the restricted space and number of possible hydrogen bond donors and acceptors, the strongly bound water molecules must extend further than one monolayer from the glucose.
A fully random conformational search has been employed to investigate the first hydration shell structure of glucose-containing from one to fifteen water molecules [4056]. Both α-glucose and β-glucose gave completed hydration shells of fifteen water molecules (shown right). The acceptor (a) and donor (d) water molecules were OH1 1d1a, OH2 1d2a, OH3 1d1a, OH4 1d2a, O5 2a, OH6 1d2a. The 15 water molecules have a total of 28 hydrogen bonds.
Neutron diffraction experiments have shown that fructose, glucose, and mannose molecules form H-bonds of different lengths and strengths that correlate somewhat with their sweetness (taste).
Sweetness
Fructose > Glucose > Mannose
Average hydrogen bond length to sugar
Fructose < Glucose < Mannose
The average hydrogen bond length to sugar was calculated considering all oxygen and hydrogen sites on the sugars and weighting by the relative concentrations of anomers [3352]. Even though the low-molecular-weight sugars have similar structures, small differences give rise to different binding strengths to water [3452]. Artificial sweeteners have a much more intense sweet taste compared to natural sugars. they do this by forming strong hydrogen bonds with many water molecules within the hydration shell and exposing a wide hydrophobic area to the receptor [4238].
The solubilities of carbohydrates in water increase with increasing temperature but reduce with increasing pressure [1892].
[Back to Top ![]() ]
]
a Although scyllo-inositol is not a monosaccharide sugar, it is used here as a more straightforward, but related example of this type of structure. [Back]
b A highly stabilized crystal structure also contributes to this low solubility. [Back]
c Favored intramolecular hydrogen bonding also contributes to the relatively poor hydration of the β-linked sugar residues [791]. [Back]
d With 'bound' being defined as having a longer relaxation time than 7.93 ps at 27 °C. [Back]
[Back to Top ![]() ]
]
Home | Site Index | Polysaccharide hydration | Nucleic acid hydration | Hofmeister effect | Kosmotropes and chaotropes | Intracellular water | LSBU | Top
This page was established in 2000 and last updated by Martin Chaplin on 29 October, 2021