
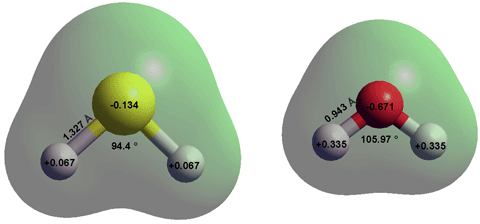
Comparison of H2S and H2O, calculated using the Restricted Hartree-Fock
wave function (RHF) using the 6-31G** basis set.
Sulfur is central to the industrial chemiical sector. It has wide redox chemistry.
Sulfur (S) has atomic number 16 with electrons (1s22s22p63s23p43d0). It can have an expanded octet because it can shift its electrons to the empty 3d orbitals in its outer shell (1s22s22p63s13px13py13pz13dxz13dyz1). As the S atom is large, it can thus form six bonds (SVI) (e.g., SF6). However, it is not large enough to accommodate six chlorine atoms, so SCl6 does not exist. The hybridization is sp3d2 and results in an octahedral shape and nonpolar character. Sulfur has several allotropes (e.g., cyclo-S8, amorphous sulfur).
Sulfur reacts with oxygen to form sulfates.
Hydrogen sulfide a (H2S) is a colorless, dense, flammable, reactive, and corrosive gas (boiling point, -60 °C; melting point, -85.5 °C, triple point, -85.49 °C, 23.1 kPa; critical temperature 100.25 °C, critical pressure 8.96 MPa, critical density 347.62 kg ˣ m−3; liquid density -60 °C, 949.0 kg ˣ m−3; surface tension -60.2 °C, 25. 43 kN ˣ m−1 r, specific heat capacity CP 26 J ˣ mol−1 ˣ K−1, entropy of vaporization 87.9 J ˣ mol−1 ˣ K−1 at boiling point) with a characteristic unpleasant and highly toxic odor of rotten eggs even at very low concentrations (> ~0.5 ppb). Dangerously, at very high concentrations, its smell is undetectable. It is naturally produced and utilized in living metabolism, f in the putrefaction of organic matter in water, and volcanic gases. It is found in amounts of about 5 μg ˣ m−3 (5 ppb) in the air. It is almost nonpolar with a dipole moment of 0.97 D in the gas phase and 1.25 D in the aqueous phase [3846]. In an aqueous solution, it acts as a weak acid. Its structure (see above) and properties are very different from H2O, in contrast to the apparent similarity, as an analog, at first glance. H2S is very soluble in common organic solvents.
H2S is only slightly soluble in water with a minimum solubility at about 200 °C (1 atm, mole fraction (H2S + SH− + S2−) = 0.0005; mole fraction at 0 °C and 25 °C being 0.00375 and 0.0019 respectively); the solubility behavior being similar to other nonpolar gases. It behaves as a weak acid. g The (earlier disputed c) high pKa2 means that the concentration of S2- in an aqueous solution is generally negligible; this was confirmed in 2018 [4452], with the statement "S2−(aq) should be expunged from the chemical literature".
H2S + H2O ![]() SH− + H3O+ pKa1 = 6.98 (25 °C) b
SH− + H3O+ pKa1 = 6.98 (25 °C) b
SH− + H2O ![]() S2- + H3O+ pKa2 = ~17 (25 °C) c
S2- + H3O+ pKa2 = ~17 (25 °C) c
The pKa1 drops to a minimum of about 6.5 at about 100 °C. e H2S is a weak reducing agent. It forms colorless solutions that turn yellow with the formation of insoluble sulfur.
S0 + 2H+ + 2e− ![]() H2S E°' = +0.17 V d
H2S E°' = +0.17 V d
S0 + H2O + 2e− ![]() HS− + OH− E°' = -0.478 V [70]
HS− + OH− E°' = -0.478 V [70]
S0 + 2e− ![]() S2− E°' = -0.508 V [70]
S2− E°' = -0.508 V [70]
2 H2S + O2 ![]() 2 S0 + 2 H2O slow (pH <~ 6)
2 S0 + 2 H2O slow (pH <~ 6)
2 HS− + O2 + 2H+ ![]() 2 S0 + 2 H2O slow (pH >~ 6)
2 S0 + 2 H2O slow (pH >~ 6)
H2S + 2 O2 ![]() HSO4− + H+ very slow
HSO4− + H+ very slow
Comparison of H2S and H2O dimers, calculated using the Restricted
Hartree-Fock wave function (RHF) using the 6-31G** basis set.
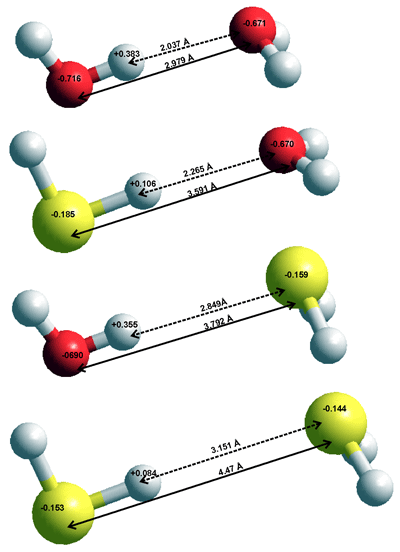
The low atomic charges (see top right), large molecular dimensions, and low polarity of H2S mean that H2S - H2O interactions, including their very weak hydrogen bonds, are far smaller than H2O - H2O interactions. This is due to the increased dispersive interactions being more than compensated by the significantly decreased electrostatic interactions. The in vacuo dimers are given on the right with interactions:
H2O···H2O ≫ HSH···OH2 > HOH···SH2 ≫ H2S···H2S
Possible hydrogen bonds between H2S molecules have been investigated. u
In H2S solutions, the water···water interactions are far stronger than the interactions involving H2S so that the mixed clusters are negligible with H2S molecules generally excluded from the water hydrogen bonding network and with the H2S preferring a dodecahedral water clathrate environment [3846].
Even if single hydrogen bonds transiently form between H2S and H2O, the H2S molecule cannot sustain any further hydrogen bonds, whereas the H2O molecules prefer having four hydrogen bonds.
Dissolved H2S acts as a powerful surfactant lying at the surface with the sulfur atom pointed away from the bulk water [3846].
[Back to Top ![]() ]
]
Sulfur dioxide
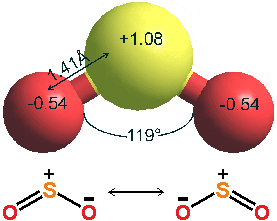
Sulfur dioxide (SO2) is a toxic pollutant gas (boiling point, 101.3 kPa, -10 °C; melting point, -75.5 °C, critical temperature 157.5 °C, critical pressure 7.88 MPa, critical density 525 kg ˣ m−3). s It is released mostly from the combustion of fuels, particularly coal and coke (~20 Gkg ˣ yr−1), and oil and gas (~9 Gkg ˣ yr−1), but also naturally from volcanoes (~20 Gkg ˣ yr−1, together with carbonyl sulfide). H2S and other biogenic sulfides such as dimethyl sulfide and carbon disulfide emitted into the air are mostly oxidized to SO2 followed by eventual oxidation (months) to H2SO4. Sulfur is removed from the troposphere in the rain, by gravitational settling, or by being directly absorbed at the surface of land or water. The most significant SO2 polluting countries are India, Russia, and China. It is a colorless, dense, non-flammable, and reactive gas with a nasty, irritating smell (boiling point, -10 °C; melting point, -72 °C, critical temperature 430.7 K, critical pressure 7.78 MPa). Its atmospheric concentration varies between good (1 ppb) and very poor (0.2 ppm) levels and is about four times that of hydrogen sulfide (the other significant atmospheric S-compound). Sulfur dioxide is primarily manufactured for sulfuric acid production. It is responsible for 'acid rain'.
SO2(g) + 2 H2O(l) ![]() HSO3−(aq) + H3O+(aq)
HSO3−(aq) + H3O+(aq)
Sulfur dioxide is formed by the burning of sulfur or materials that contain sulfur,
S2 (g) + 2 O2 (g) → 2 SO2 (g) ΔH = -723 kJ ˣ mol−1 at 298 °C,
2 H2S (g) + 3 O2 (g) → 2 H2O + 2 SO2 (g)
In the atmosphere, the following reactions have been proposed [3985],
1SO2 (g, singlet) + UV → 3SO2 (g, triplet)
3SO2 (g, triplet) + H2O (g) → OH• (g) + HOSO• (g)
1SO2(g) + OH• (g) → HOSO2• (g)
HOSO2• (g) + O2 (g) → SO3 (g) + HOO• (g)
SO3 (g) + H2O (g) → H2SO4 (g)
Sulfite results from the action of an aqueous base on sulfur dioxide,
SO2 + NaOH → NaHSO3
SO2 + 2 NaOH → Na2SO3 + H2O
Sulfuric acid is made industrially by further oxidation by the 'contact process' utilizing vanadium(V) oxide (V2O5) catalyst.
2 SO2 + 2 H2O + O2 → 2 H2SO4
The ice snowpack is a highly efficient sink for atmospheric SO3 depletion, especially in winter [3996].
The bisulfite ion has an equilibrium structure, Keq = 0.2 o
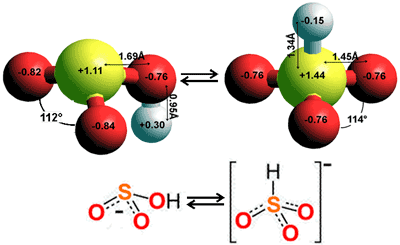
The aqueous chemistry of the oxides of sulfur and related acids is very complex, involves at least eight sulfur oxidation states, and is not restricted by the 'octet rule'. Sulfur dioxide (SO2) and sulfur trioxide (SO3) are the acid anhydrides of sulfurous acid (H2SO3) and sulfuric acid (H2SO4), respectively, and are both very soluble in water. Sulfurous acid cannot be isolated from aqueous solutions but forms bisulfites and is stable in the gas phase. p
SO2(g) ![]() SO2(aq)
SO2(aq)
SO2(aq) + H2O ⇌ SO2.H2O
SO2.H2O + H2O ⇌ HSO3− + H3O+
H2SO3 is a strong acid (pKa = 1.86). HSO3− is weakly acidic (pKa = 6.97) with the equilibrium above lying mostly towards hydrates SO2 (SO2.H2O, Keq ≪ 10−9). Raman spectra of sulfur dioxide solutions in water show only signals due to the SO2 molecule and the bisulfite ion. Because of its low concentration, the bisulfite dissociates to the sulfite ion, SO32−.
SO2.H2O, calculated using the Restricted
Hartree-Fock wave function (RHF) using the 6-31G** basis set
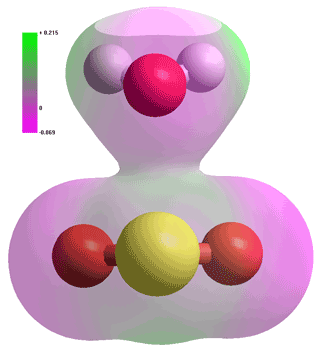
HSO3− + H2O ![]() SO32− + H3O+
SO32− + H3O+
Many bisulfites form metabisulfites (disulfites) on drying
2 HSO3− ⇌ S2O52− + H2O
The photochemistry of SO2 at the air-water interface of water droplets leads to the formation of the strongly acidic (pKa = -1) HOSO• radicals that may play an important role in acid rain formation [3978].
Putative disulfurous acid; like sulfurous acid
is another phantom acid
Many oxy-sulfur anions contain expandable S-S bonds (1.8 - 3.0 Å) that are flexible with bond angles between 90° and 180° and dihedral angles between 0° and 180°. n
The metabisulfite anion consists of an SO2 group linked to an SO3 group, with an S-S bond (2.22 Å m) and the negative charges localized on both ends. Such structures can exist as both [2OSOSO2]2− (see above right l) and [3OS-SO2]2− forms, with [3OS-SO2]2− found in crystals but with inequivalent ions. j The thermal decompositions of the sodium-sulfur oxo-salts have been examined. k
Redox chemistry of sulfur showing some of the oxidation states h
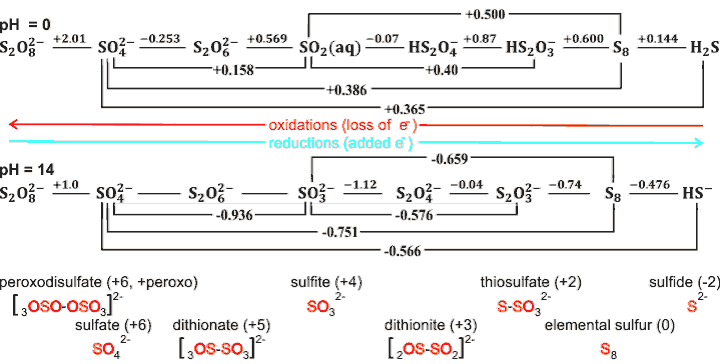
Peroxodisulfate (S2O82−, persulfate) is a vpowerful oxidizing agent i used as a polymerization initiator. It can be serially reduced, producing the intermediates shown above. It decomposes to give sulfate radical anion or hydrogen peroxide,
S2O82−![]() 2 SO4•−
2 SO4•−
S2O82−+ 2H2O → 2HSO4− + H2O2
The strongest reducing agent is dithionate, followed by sufite.
Sulfurous (left) and sulfuric acids, calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set
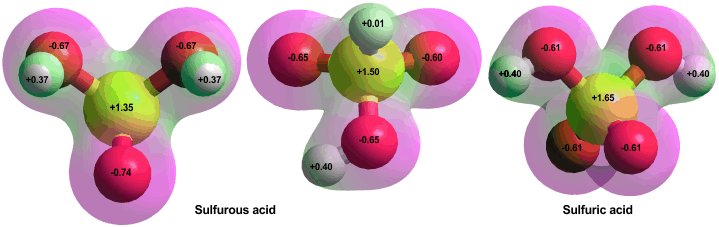
There are two possible forms of sulfurous acid (far left and middle, above). Three isomers of the left-hand structure are cis-cis (the most stable, shown), cis-trans (3.8 kJ ˣ mol−1 less stable), and trans-trans (21 kJ ˣ mol−1 less stable). v Modeling does give a stable (if more energetic) structure with an S-H bond similar to that in the bisulfite ion equilibrium (see middle above). Sulphuric acid (right above), but not sulfurous acid, is stable in aqueous solutions.
[Back to Top ![]() ]
]
Sulfur trioxide
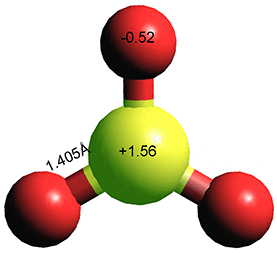
Sulfur trioxide (SO3) is a nonpolar trigonal planar molecule where the sulfur is in its hexavalent state (+6). Sulfur trioxide (SO2) is a corrosive gas (boiling point, 101.3 kPa, 44.8 °C; melting point, +16.86 °C for orthorhombic γ-SO3, critical temperature 217.7 °C, critical pressure 8.19 MPa) s that gives rise to acid rain. An equilibrium exists between the monomer, SO3, and its trimer, S3O9, with its equilibrium shifted towards the trimer at low temperatures in the gaseous and liquid phases. In the presence of trace amounts of water (~ 100 ppm), SO3 converts to HO(SO2O)xH whiskers below ~27 °C.
The catalytic oxidation of sulfur dioxide creates sulfur trioxide,
2 SO2 + O2 ![]() 2 SO3 ΔH° = -99 kJ ˣ mol−1
2 SO3 ΔH° = -99 kJ ˣ mol−1
SO3 trimer
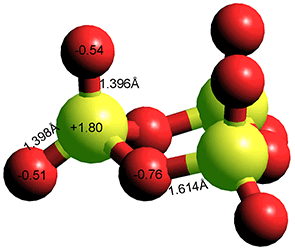
Industrially, the gaseous sulfur trioxide is then absorbed in sulfuric acid, where it reacts with added water to form more sulfuric acid.s
H2SO4 + SO3 ![]() H2S2O7
H2S2O7
H2S2O7 + H2O ![]() 2 H2SO4
2 H2SO4
Reaction with just water should be avoided as SO3 is aggressively hygroscopic, with its reaction being extremely violent.
SO3 + H2O ![]() H2SO4
H2SO4
SO3 hydration prefers more than one water molecule [4205]. See below.
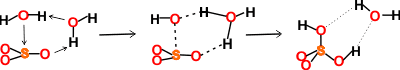
The SO3 hydration mechanism at the air-water interface of water droplets has been investigated [4201]. After uptake at the interface, the SO3 reacts rapidly with water molecules to form ion pairs of HSO4− and H3O+ or molecular H2SO4 within a few picoseconds.
[Back to Top ![]() ]
]
Sulfuric acid (see above right, boiling point, 279.6 °C where it slowly decomposes; melting point, -10.4 °C; density 1835.6 kg ˣ L−1, 15 °C) t is a clear, polar (dielectric constant = 101 25 °C q), colorless, odorless, highly corrosive, and slightly viscous liquid. It is very hygroscopic, miscible with water (highly exothermic), and strongly oxidizing and dehydrating, for example, removing water from sugars (charring), converting them to carbon and steam, and producing a low density, solid, black substance including gas bubbles. Sulfuric acid is widely used in the chemical industry and in lead-acid batteries. Concentrated H2SO4 is usually 98.3 % by mass (18.4 mol ˣ L−1), and lead battery acid is about 30 % by mass (4.5 mol ˣ L−1). Pure sulfuric acid is ionized to a small extent, giving a moderate conductivity (0.01 S ˣ cm−1),
H2SO4 + H2SO4 ![]() HSO4− + H3SO4+
HSO4− + H3SO4+
H2SO4 + H2SO4 ![]() H2S2O7− + H3O+
H2S2O7− + H3O+
Diluted in water, it acts as a strong dibasic acid, with acid dissociation constants (see also for Hammett description),
H2SO4 + H2O ![]() HSO4− + H3O+ pKa1 = -6.4
HSO4− + H3O+ pKa1 = -6.4
HSO4− + H2O ![]() SO42− + H3O+ pKa2 = +1.98
SO42− + H3O+ pKa2 = +1.98
but above 7 mol ˣ L−1, there are sulfate–hydronium ion pairs [H3O+.SO42−] and ion triplets [H3O+.SO42−.H3O+] [4005]. The more negative charge and hence stronger repulsions in the SO42− ion leads to a larger diameter (average S=O bond length 1.49 Å) than HSO4− (average S=O bond length 1.44 Å). On ab initio optimization of the sulfate ion (SO42− ), the tetrahedrally-placed atomic charges are, S = +1.498, and O = −0.875 with S-O = 0.1487 nm. These values may be compared with the parameters chosen for the TIP4P/2005 tetrahedral model of S = +0.9, and O = −0.65 with S-O = 0.149 nm [984c] (this model includes scaling of the coulombic interactions to account for the screening effect of the electronic continuum).
Sulfuric acid has high electrical conductivity due to its autoprotolysis,
H2SO4 + H2SO4 ![]() HSO4− + H3SO4+ pK = 3.6
HSO4− + H3SO4+ pK = 3.6
This is about 1010 greater than the ionization of water (Kw = 10−14)
Disulfuric acid
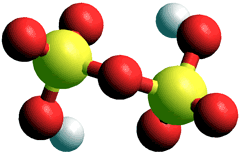
Pure sulfuric acid reacts with sulfur trioxide to form oleum (SO3)y.H2SO4; y = 0 - 1, also called fuming sulfuric acid, with the fumes mostly SO3; a mixture of sulfuric and disulfuric acids. If completely converted to H2S2O7 (i.e., y = 1), it is also called disulfuric acid or pyrosulfuric acid (melting point 36 °C) that reacts with water to reform H2SO4, This is the critical final step in the 'Contact Process' for the production of sulfuric acid.
H2SO4 (anhydrous, pure) + SO3 ![]() H2S2O7
H2S2O7
H2S2O7 + H2O ![]() 2 H2SO4
2 H2SO4
[Back to Top ![]() ]
]
a The spellings 'hydrogen sulphide', sulphur dioxide' and 'sulphur' (etc.) are not recommended [IUPAC] but still acceptable. Nowadays the 'ph' in 'sulphur' compounds should always be replaced by 'f'. [Back]
b F. J Millero, The thermodynamics and kinetics of the hydrogen sulfide system in natural waters, Marine Chemistry, 18 (1986) 121-147. [Back]
c W. Giggenbach, Optical spectra of highly alkaline sulfide solutions and the second dissociation constant of hydrogen sulfide. Inorganic Chemistry, 10 (1971) 1333-1338; this high value is disputed with others claiming a value around pKa2 = 14, R. S., Ramachandra and L. G. Hepler, Equilibrium constants and thermodynamics of ionization of aqueous hydrogen sulfide. Hydrometallurgy, 2 (1977) 293-299. [Back]
d O. Kabil and R. Banerjee, Redox biochemistry of hydrogen sulfide, Journal of Biological Chemistry, 285 (2010) 21903-21907. [Back]
e J. A. Barbero, K. G. Mccurdy and P. R. Tremaine, Apparent molal heat capacities and volumes of aqueous hydrogen sulfide and sodium hydrogen sulfide near 25°C: the temperature dependence of H,S ionization, Canadian Journal of Chemistry, 60 (1982) 1872-1880. [Back]
f E. Cuevasanta, M. N. Möller and B. Alvarez, Biological chemistry of hydrogen sulfide and persulfides, Archives of Biochemistry and Biophysics, 617 (2017) 9-25. [Back]
g The pKas of H2Se and H2Te are 3.7 and 2.7 respectively [3888b], with that for H2Te thirteen orders of magnitude greater than that for H2O. [Back]
h N. Wiborg, Inorganic Chemistry, Academic Press, San Diego, 2001, ISBN 0-12-352651-5. [Back]
i S. A. Shafiee, J. Aarons and H. H. Hamzah. , Review—Electroreduction of peroxodisulfate: A review of a complicated reaction, Journal of The Electrochemical Society, 165 (2018) H785-H798 [Back]
i K. L. Carter, T. A. Siddiquee, K. L. Murphy and D. W. Bennett, The surprisingly elusive crystal structure of sodium metabisulfite, Acta Crystallographica Structural Science, B60 (2004) 155-162. [Back]
k B. Jaszczak-Figiel and Z. Gontarz, Stages of thermal decomposition of sodium oxo-salts of sulfur, Journal of Thermal Analysis and Calorimetry, 96 (2009) 147-154. [Back]
l M. Abedi, M. Vahedpour, S. Farnia and H. Farrokhpour, Theoretical study on the structures, stabilities and electronic properties of S2O52− isomers in the gas and solution phases, Molecular Physics: An International Journal at the Interface Between Chemistry and Physics, 111 (2013) 581-588. [Back]
m B. I-C. Chen and Y. Wang, Reinvestigation of the structure of potassium pyrosulfite, K2S2O5, Acta Crystallographica, C40 (1984), 1780-1781. [Back]
n R. Steudel, Properties of sulfur-sulfur bonds, Angewandte Chemie, 14 (1975) 655-720. [Back]
o D. A. Hornert and R. E. Connick, Equilibrium quotient for the isomerization of bisulfite ion from HS03−to S03H−, Inorganic Chemistry, 25 (1986) 2414-2417. [Back]
p D. Siilzle, M. Verhoeven, J. K. Terlouw and H. Schwarz, Generation and characterization of sulfurous acid (H2SO3) and of its radical cation as stable species in the gas phase, Angewandte Chemie International Edition, 27(1988) 1533-1534; E. Bishenden and D. J. Donaldson, Ab initio Study of SO2+H2O, Journal of Physical Chemistry A, 102 (1998) 4638-4642. [Back]
q R. J. Gillespie and R. H. Cole, The dielectric constant of sulphuric acid, Transactions of the Faraday Society, 52 (1956) 1325-1331. [Back]
r F. Pouliquen, C. Blanc, E. Arretz, I. Labat, J. Nougayrede, G. Savin. R. Ivaldi, M. Nicolas, J. Fialaire, R. Millischer, C. Azema, L. Espagno, H. Hemmer, and J. Perrot, Hydrogen sulfide, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Vol. 18 (2012) pp 429-449, DOI: 10.1002/14356007.a13_467. [Back]
s H. Müller, Hydrogen sulfide, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Vol. 35 (2012) pp 73-118, doi:10.1002/14356007.a25_569. [Back]
t H. Müller, Sulfuric acid and sulfur trioxide, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Vol. 35 (2012) pp 141-211, doi:10.1002/14356007.a25_635. [Back]
u J. R. Sabin, Hydrogen bonds involving sulfur. I. The hydrogen sulfide dimer, Journal of the American Chemical Society, 93 (1971) 3613-3620. [Back]
v S. Mallick, A. Kumar and P. Kumar, Kinetic instability of sulfurous acid in the presence of ammonia and formic acid, Physical Chemistry Chemical Physics, 22 (2020) 18646-18654. [Back]
[Back to Top ![]() ]
]
Home | Site Index | Aqueous phosphate | Aqueous ammonia | Aqueous hydrogen halides | LSBU | Top
This page was established in 2020 and last updated by Martin Chaplin on 1 June, 2022