
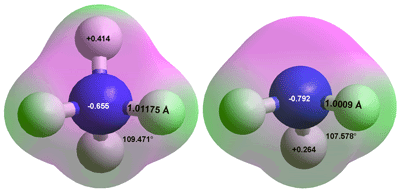
Ammonium, NH4+ Ammonia, NH3
Ammonia is central to industrial chemistry and metabolic biochemistry.
![]() Nitrogen oxides | N2O | NO | NO2
Nitrogen oxides | N2O | NO | NO2
![]() Nitrous and nitric acid
Nitrous and nitric acid
Ammonia (NH3) is a colorless flammable gas (boiling point, -33.34 °C; melting point, -77.73 °C; liquid density, 638.6 kg ˣ m−3 (at 0 °C, 101.3 kPa); critical temperature 132.22 °C, critical pressure 11.345 MPa, critical density 234.3 kg ˣ m−3 [3685], 17.030 26 g ˣ mol−1 [IAPWS], 14NH3 99.5872 %; 15NH3 0. 366 129 %; 14NH2D 0. 046 %). Ammonia is primarily formed by the decomposition of nitrogen-containing organic materials or through volcanic activity. f Pure ammonia dissociates like water, showing significant electrical conductance,
NH3 + NH3 ![]() NH2− + NH4+
NH2− + NH4+
Unlike liquid water, which optimally possesses four stronger hydrogen bonds per oxygen atom, each nitrogen atom in liquid ammonia is found to have only one weaker hydrogen bond [4476]. Also, in contrast to crystalline water-ice, hydrogen bonding is practically nonexistent in crystalline ammonia-ice. In liquid ammonia, there is no clear peak for hydrogen-bonded N···H in the radial distribution function N−H(r). Hydrogen bonding is practically nonexistent in crystalline ammonia, and in contrast to the behavior of water, crystalline ammonia is 10% denser than liquid ammonia.
Ammonia is one of the world's most significant manufactured chemicals (primarily for fertilizer) after sulfuric acid. The Haber process manufactures it, k where a mixture of H2 (from natural gas) and N2 (from the air) reacts over a catalyst (often iron) at elevated temperature (400°C – 500°C) and pressures (>10 MPa). e Although the reaction is exothermic, a high activation energy must be overcome for the triple-bonded nitrogen molecule to be split.
N2 (g) + 3 H2 (g) ![]() 2 NH3 (g) ΔH° = -46.22 kJ ˣ mol−1 at 25 °C
2 NH3 (g) ΔH° = -46.22 kJ ˣ mol−1 at 25 °C
Ammonia is very soluble in water (it is the most soluble gas), where it reacts to form ammonium ions and hydroxide ions at appropriate pH. Its commonly available concentrated solution has a density of 880 kg ˣ m−3 (35.6 % ammonia) at 15 °C. In an aqueous solution, ammonia is a weak base and a general-purpose cleaner. Ammonia–water complexes, where NH3 molecules are weakly hydrogen-bonded to surface water molecules, are found on the surface of aqueous ammoniacal solutions [3687]. A saturated solution is highly corrosive and contains about 0.31 kg ammonia per kg solution at 25 °C, and has a density of about 0.88 g ˣ mL−1 (> 13 M). Solutions give off ammonia gas, and concentrations are approximate. These species are shown in the above right figure (C3ν)a where all the hydrogen atoms in both structures are positioned equivalently. The ammonia molecule may invert (like a wind-blown umbrella, see also the hydrogen ion) through the planar transition state (C3h). The two equilibrium structures of ammonia are superimposable and indistinguishable, with the molecules symmetrically distributed between the two potential wells, at all times [4326]. The inversion has an energy barrier in vacuo of about 24 kJ ˣ mol−1 [3686], but in contrast to the H3O+ ion, acceptor hydration at the nitrogen atom prevents the inversion. Ammonia inversion is described perfectly by the tunneling of the p orbital through the molecular plane [4001]. The frequency of the oscillation is about 200 THz. The thermodynamic properties of ammonia-water mixtures have been reported [IAPWS, 3684].
H2O + NH3 ![]() OH− + NH4+ pKb (NH3) = 4.755 at 25 °C [3681]
OH− + NH4+ pKb (NH3) = 4.755 at 25 °C [3681]
H2O + NH4+![]() H3O+ + NH3 DH° = +51.92 kJ ˣ mol−1 at 25 °C [3680]
H3O+ + NH3 DH° = +51.92 kJ ˣ mol−1 at 25 °C [3680]
pKa (NH4+) = -Log10([H3O+] ˣ [NH3]/ [NH4+]) = 9.245 at 25 °C
pH = 9.245 + Log10([NH3]/ [NH4+]) at 25 °C
A 1 M solution of NH4OH has a pH of 11.77 at 18 °C. The relationship of pKa (NH4+) to temperature is pKa (NH4+) = 0.09018 + 2729.92/T where T is in Kelvin [3681].
Ammonium (NH4+) is a very weak acid, with an equilibrium constant (5.7 ˣ 10−10 M) approximately 70 times lower than physiological (buffered) hydrogen ion concentration (4.0 ˣ 10−8 M), so that when ammonia is produced in the body, it is immediately mostly converted (~98 %) to ammonium ions. However, when added to pure unbuffered water it mostly remains as dissolved NH3.
Ammonia is a reducing agent and can be produced by the electro-synthesis of ammonia [3871]:
N2 + 6 H+ + 6 e−![]() 2 NH3 E° = +0.550 V
2 NH3 E° = +0.550 V
N2+ 2 H2O + 6 H+ + 6 e− ![]() 2 NH4 OH E° = +0.092 V
2 NH4 OH E° = +0.092 V
N2 + 8 H+ + 6 e− ![]() 2 NH4+ E° = +0.270 V
2 NH4+ E° = +0.270 V
Ammonia may be oxidized 4 NH3 (g) + 3 O2 (g) ![]() 2 N2 (g) + 6 H2O (g)
2 N2 (g) + 6 H2O (g)
or, if catalyzed by platinum ,
4 NH3 (g) + 5 O2 (g) ![]() 4 NO (g) + 6 H2O DH° = -905.2 kJ ˣ mol−1
4 NO (g) + 6 H2O DH° = -905.2 kJ ˣ mol−1
This oxidation is the first stage of the Ostwald process, under pressure (~ 0.4 - 1.0 MPa) and temperature (~600 - 800 °C), used for making nitric acid (HNO3)
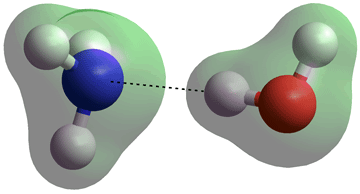
H3N···H-OH hydrogen bond in the gas phase
cis- and trans- H3N···H-OH hydrogen bonds
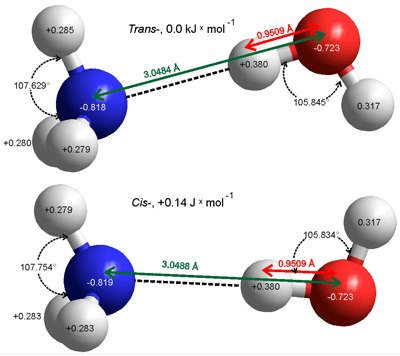
When attached to the water molecule, both the ammonia and water molecules lose some of their symmetry and the ammonia molecule cannot invert. The two very shallow energy minima cis- and trans- structures of the H2N-H ···OH2 hydrogen bond in the gas phase are given on the left. The energy difference (0.14 J ˣ mol−1) is not significant when compared with that of the hydrogen bond (25 kJ ˣ mol−1) and the thermal energy (2.5 kJ ˣ mol−1) leaving the H2O and NH3 molecules to rotate freely around the hydrogen bond. The (partially constrained) molecular parameters have also been determined with the CCSD(T)-F12A method and the VTZ-F12 basis sets with similar results. [3682]
H3N+-H···OH2 hydrogen bond in the gas phase
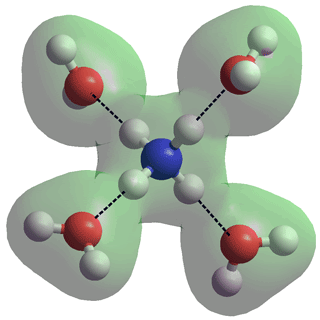
In an aqueous solution, both NH3 and NH4+ are hydrated. NH4+ can form four strong, tetrahedrally-placed and long-lived, donor hydrogen bonds to H2O molecules [136] (see right, N-H, 1.01686 Å, N···O, 2.90274 Å). This structure is much more stable than a cluster with an NH3 in the center and an H3O+ on the surface [194]. NH3 only forms one moderately-strong acceptor hydrogen bond (see the NH3 ···OH2 cis-/trans- figure, above left) with the three much weaker donor H2N-H···OH2 (ultra-weak) hydrogen bonds losing out to the much stronger HO-H···OH2 interactions [3688]. In the magic ion NH4+(H2O)20, the NH4+ ion sits centrally in the water dodecahedron with the four hydrogen bonds from the central ammonium ion equivalent (see the connectivity map), so helping to explain the apparent increased structural stability of this ion relative to H3O+(H2O)20. The exchange of hydrogen bond partners by the central NH4+ explains the faster than expected rotation [855].
Note that a single H2O hydrogen-bonded NH4+ ion (H3N+-H···OH2) forms one of the strongest hydrogen bonds known at 92.5 kJ ˣ mol−1 [447]. In this structure, the hydrogen bond H3N+-H···OH2 shrinks by 0.15 Å (and the N-H covalent bond expands by 0.018 Å) between the tetra-coordinated and the single-coordinated NH4+, in line with the stronger bonding of the latter.
Under high pressures, such as occuring in large planetary systems like Neptune and their moons such as Titan, Callisto, and Ganymede, mixtures of water and ammonia may form a complex phase diagram with many crystal forms [3683]. The multiplicity of these is not only due to the same reasons as for the complexity of the water phase diagram but plus the additional complexity introduced by the multi-component-system present. A total of twelve mixed phases exist containing both NH3 and H2O molecules under various pressure-temperature conditions; ammonia monohydrate (NH3:H2O = 1:1, six phases), ammonia dihydrate (NH3:H2O, 1:2, four phases), and ammonia hemihydrate (NH3:H2O, 2:1, two phases) [3861]. Using classical molecular dynamics simulations, the rotational relaxation of ammonia is approximately three times faster than water in mixed solutions, which further increases along with concentration and pressure [3914]. Also, the water-water and water-ammonia hydrogen-bond lifetimes increase with ammonia concentration, whereas they pass through shallow maxima with pressure.
[Back to Top ![]() ]
]
Ammonia lies at the root of the nitrogen redox series [70],
reductant
N2 + 6 H+ + 6 e−![]() 2 NH3 E° = +0.550 V
2 NH3 E° = +0.550 V
N2O + 2 H+ + 2 e−![]() N2 + H2 E° = +1.766 V
N2 + H2 E° = +1.766 V
2 NO + 2 H+ + 2 e− ![]() N2O + H2O E° = +1.591 V
N2O + H2O E° = +1.591 V
HNO2 + H+ + e− ![]() NO + H2O E° = +0.983 V
NO + H2O E° = +0.983 V
NO3− + 3 H+ + 2 e− ![]() HNOc + H2O E° = +0.934 V
HNOc + H2O E° = +0.934 V
oxidant
Nitrous oxide (N2O, also but rarely called dinitrogen oxide) is a soluble, i colorless, anesthetic gas (often was used in dentistry, and now used as an abused recreational drug). N2O, is also known as 'laughing gas' (boiling point, -88.48 °C; melting point, -90.86 °C; liquid density, 793 kg ˣ m−3 (at 20 °C, 101.3 kPa); critical temperature 36.41 °C, critical pressure 7.245 MPa, critical density 452 kg ˣ m−3).g It is an oxidant (e.g., oxidizing benzene to phenol) and may be used in rocket propellants and racing car fuels. It is also used as the propellant in canned whipped cream, dissolving well in the cream. Unusually, it is polar and hydrophobic, dissolving best in cold water and preferring not to form hydrogen bonds with water.
.
Nitrous oxide d is a major scavenger of atmospheric ozone (O3). It substantially contributes to global warming, with annually increasing atmospheric concentrations (up 20% from ~270 ppb by volume in 1800 to more than 322 ppb today due to fertilizer use and nitrogen-fixing crops). c It has a significant atmospheric lifetime with a loss of 63.2% every 114 years.
N2O + hv → N2 + O
N2O + O → N2 + O2
N2O + O → 2 NO
2 NO + O2 → 2 NO2
NO2 + O → NO + O2
NO + O3 → NO2 + O2
O + O3 → O2 + O2
Nitrous oxide is produced naturally (~11.5 ˣ 109 ˣ kg ˣ yr−1), mainly in the soil and linked to agriculture by the bacterial and archaeal oxidation of ammonium to nitrite (nitrification, ammonium monooxygenase, ![]() NH2OH, and hydroxylamine oxidoreductase,
NH2OH, and hydroxylamine oxidoreductase, ![]() NO2−). After partial oxidization to nitrate (nitrite oxidoreductase,
NO2−). After partial oxidization to nitrate (nitrite oxidoreductase, ![]() NO3−), it is reduced by denitrifiers to nitrous oxide (nitrate reductase
NO3−), it is reduced by denitrifiers to nitrous oxide (nitrate reductase ![]() NO2−, nitrite reductase
NO2−, nitrite reductase ![]() NO, nitric oxide reductase
NO, nitric oxide reductase ![]() N2O). The nitrogen cycle is completed by nitrous oxide reductase (
N2O). The nitrogen cycle is completed by nitrous oxide reductase (![]() N2) and nitrogenase (N2
N2) and nitrogenase (N2 ![]() NH3). b
NH3). b
The nitrogen cycle
NH4+ ![]() NH2OH
NH2OH ![]() NO2−
NO2− ![]() NO3−
NO3− ![]() NO2−
NO2− ![]() NO
NO ![]() N2O (
N2O (![]() N2
N2 ![]() NH3)
NH3)
Nitrous oxide is manufactured industrially by heating ammonium nitrate while avoiding its dissociation to ammonia and nitric acid.
useful exothermic decomposition NH4NO3 ![]() N2O + 2 H2O DH = -59 kJ ˣ mol−1, at 250 °C
N2O + 2 H2O DH = -59 kJ ˣ mol−1, at 250 °C
wasteful endothermic dissociation NH4NO3 ![]() NH3 + HNO3 DH = +159.9 kJ ˣ mol−1, at 250 °C
NH3 + HNO3 DH = +159.9 kJ ˣ mol−1, at 250 °C
NH4NO3 is a highly water-soluble oxidant and may be explosive when mixed with combustible material.
Nitric oxide (·NO), also called nitrogen monoxide, has a double bond and a 3-electron bond. It iis a colorless, toxic, light, non-flammable gas at room temperature (boiling point, -151.77 °C; melting point, -163.65 °C; critical temperature -93 °C, critical pressure 6.485 MPa, critical density 520 kg ˣ m−3).g On contact with oxygen, it oxidizes to a brown vapor (nitrogen dioxide). Nitric oxide is generated in thunderstorms from the rapid recombination of dissociated O2 and N2 within the 2-3 cm wide 30,000 °C lightning strike. It also forms in combustion engines.
Nitric oxide is produced industrially by passing air through an electric arc (raising its temperature to ~3000 °C), where the
oxygen and nitrogen,
N2 + O2![]() 2 ·NO
2 ·NO
or it can be synthesized by the catalytic oxidation of ammonia with atmospheric oxygen; the first step in the Ostwald process j for the production of nitric acid, in the presence of a catalyst, such as platinum with 10% rhodium,
4 NH3 + 5 O2![]() 4 ·NO + 6 H2O DH = -904 kJ ˣ mol−1
4 ·NO + 6 H2O DH = -904 kJ ˣ mol−1
·NO has one unpaired electron, making it a free radical that avidly reacts with other radicals,
·NO + ·NO![]() N2O2 DH = -10.5 kJ ˣ mol−1
N2O2 DH = -10.5 kJ ˣ mol−1
·NO + ·NO2 ![]() N2O3 DH = -40.58 kJ ˣ mol−1
N2O3 DH = -40.58 kJ ˣ mol−1
2 ·NO (g) +
3O2 (g) ![]() 2 ·NO2 (g) DH = -112.7 kJ ˣ mol−1
2 ·NO2 (g) DH = -112.7 kJ ˣ mol−1
This last reaction is the second step in the Ostwald process for the production of nitric acid.
·NO acts as a biological messenger in the smooth muscle and neurons of mammals. As a result, it regulates all aspects of our lives from walking, digestion, sexual function, pain perception, and pleasure, memory recall, and sleeping. h The anti-impotence drug Viagra (sildenafil) exploits the signaling role of NO and increases blood flow into the penis.
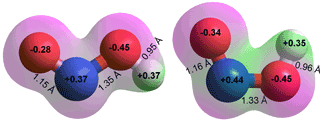
Nitrogen dioxide (·NO2) is a reddish-brown gas. (boiling point, 21.15 °C; melting point, -11.2 °C; liquid density, 1446.8 kg ˣ m−3 (at 20 °C, 101.3 kPa); critical temperature 157.85 °C, critical pressure 10.132 MPa, critical density 550 kg ˣ m−3).g ·NO2 is formed from a vehicle, power plant, and other industrial emissions. ·NO2 has one unpaired electron, making it a free radical that avidly reacts with other radicals. It dimerizes to form a planar colorless gas (dinitrogen tetroxide, N2O4 with no unpaired electrons), then a colorless liquid at low temperatures. Dinitrogen tetroxide can be prepared in two structures (see below right). The structure to the left exists at room temperature and is in equilibrium with the one on the right at low temperatures,

2 ·NO2 ![]() N2O4 DH = -58.08 kJ ˣ mol−1
N2O4 DH = -58.08 kJ ˣ mol−1
·NO + ·NO2 ![]() N2O3 DH = -40.58 kJ ˣ mol−1
N2O3 DH = -40.58 kJ ˣ mol−1
Resonance structure of ·NO2

NOx, used in atmospheric science, refers to a mixture of NO and NO2 (where x lies between 1 and 2) and is produced in vehicle exhausts and lightning strikes, after the partial combination of oxygen and nitrogen. NOx contributes to atmospheric pollution, smog, acid rain, and global warming.
·NO2 is made by the reaction of nitric oxide with oxygen (see above), but above 150 °C, it begins to dissociate,
2 ·NO2 (g) ![]() 2 ·NO (g) + O2 (g)
2 ·NO (g) + O2 (g)
Other nitrogen oxides are dinitrogen trioxide (N2O3), dinitrogen tetroxide (N2O4), dinitrogen pentoxide (N2O5), nitrogen trioxide (NO3), dinitrogen hexoxide (N2O6), and trinitramide (N(NO2)3).
Nitrogen oxides
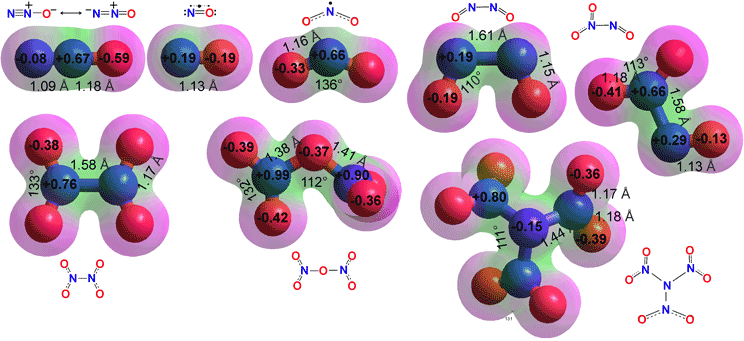
At aqueous surfaces ·NO2 dimerizes, isomerizes, hydrates, and hydrolyzes to give nitric and nitrous acids, probably further catalyzed by other materials within the clouds [4181],
+ 2H2O
2 ·NO2 ![]() O2N-NO2
O2N-NO2 ![]() O2N-O-N-O
O2N-O-N-O ![]() (O2N-O)−····(N-O)+
(O2N-O)−····(N-O)+ ![]() H3O+ + NO3− + HO-N-O
H3O+ + NO3− + HO-N-O
In the air, ·NO2 reacts with the iodine monoxide radical (·IO, enriched in marine aerosols) to form iodine nitrate (IONO2) [4009]. The iodine atom of IONO2 interacts with locally present aerosol IO3 through halogen bonding.
[Back to Top ![]() ]
]
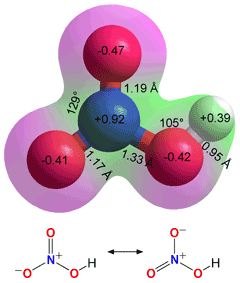
Nitrous acid exists in
two isomeric forms

cis- and trans-conformers of Nitrous acid,
cis nitrous acid is 2.83 kJ ˣ mol−1 less stable
Nitrous acid (HNO2) is a weak aqueous acid (pKa = 3.398). The acid is stable only in cold dilute
aqueous solution.
Nitrous acid can be made by the acidification of aqueous nitrites or by the solution of dinitrogen trioxide,
Ba(NO2)2 + H2SO4 ![]() 2 HNO2 + BaSO4
2 HNO2 + BaSO4
N2O3 + 2 H2O ![]() 2 HNO2
2 HNO2
Nitrous acid is stored cold and on heating, it disproportionates as,
3 HNO2 ![]() HNO3 + 2 ·NO + H2O
HNO3 + 2 ·NO + H2O
2 HNO2 ![]() N2O3 + H2O
N2O3 + H2O
In the troposphere, nitrous acid undergoes photolysis to produce radicals,
HONO + hν ![]() ·NO + ·OH
·NO + ·OH
An especially important property of nitrous acid is its ability to diazotize primary organic amines to form diazonium salts that are used widely in the manufacture of azo dyes
R-NH3+Cl− + HNO2 ![]() R-N≡N+Cl−+ 2 H2O
R-N≡N+Cl−+ 2 H2O
Nitrite (NO2−) and nitrate (NO3−) ions are compared elsewhere.
[Back to Top ![]() ]
]
Nitric acid
Nitric acid is a colorless, acrid, highly corrosive, strong acid (pKa = -1.38), and strong oxidizing agent. Although nitric acid completely dissociates at high dilution, at medium concentrations, it dissociates incompletely with some molecules (HNO3) still present, and it ultimately remains in its molecular form from about an equimolar composition onwards [4005]. However, it dissociates incompletely with some molecules (HNO3) still present. Pure anhydrous nitric acid is dense (1512.8 kg ˣ m−3 at 20 °C; ~24 M,; boiling point 86 °C; melting point -42 °C, 23.867 mol ˣ L−1), g and at 69.2 wt %, it forms a maximum-boiling azeotrope boiling at 121.8 °C. However, it is difficult to obtain pure nitric acid due to its unstable chemical behavior, and it generally contains traces of NO3−, NO2+, and H3O+ ions, water, and dissolved NO2 [4005].
Nitric acid dimer
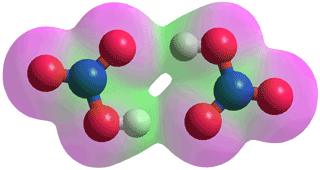
Nitric acid forms dimers (see left)
The final stages of the Ostwald process used for making HNO3 are,
2 ·NO (g) + O2(g) ![]() 2 ·NO2 (g) ΔH = −114 kJ ˣ mol−1
2 ·NO2 (g) ΔH = −114 kJ ˣ mol−1
3 ·NO2 (g) + H2O (l) ![]() 2 HNO3 (aq) + ·NO (g)
2 HNO3 (aq) + ·NO (g)
ΔH = −117 kJ ˣ mol−1
The overall reaction for the Ostwald process is, generating much heat,
2 NH3 (g) + 4 O2 (g) + H2O (l) ![]() 3 H2O (g) + 2 HNO3 (aq) ΔH = −740.6 kJ ˣ mol−1
3 H2O (g) + 2 HNO3 (aq) ΔH = −740.6 kJ ˣ mol−1
Hydrated nitric acid held by strong H-bonds
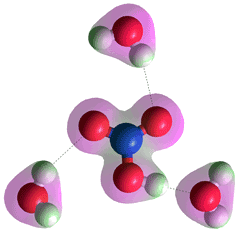
Polar stratospheric cloud layers are found at heights between 20-24 km at atmospheric temperatures between 185 K to 197 K. Analysis shows that a significant fraction of these cloud layers was composed of nitric acid trihydrate particles that can lead to ozone destruction [4004]. Atmospheric nitrogen oxides NOx, Vfrom volcanic eruptions, industrial and vehicular emissions, and degradation of biological substances, hydrate to deplete atmospheric ozone and form HNO3 in the troposphere and stratosphere, then precipitated in acid rain [4231].
HNO3 decomposes slowly or on heating, giving off brown fumes of NO2, and turning the liquid yellow,
4 HNO3 ![]() 2 H2O + 4 ·NO2 + O2
2 H2O + 4 ·NO2 + O2
[Back to Top ![]() ]
]
a The molecular structures on this page were calculated in the gas phase, using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. This is in line with other calculated structure factors given on this website (e.g., the values for the water molecule and its clusters). [Back]
b D. E. Canfield, A. N. Glazer and P. G. Falkowski, The evolution and future of Earth's nitrogen cycle, Science, 330 (2010) 192-196; J. I. Prosser, L. Hink, C. Gubry-Rangin and G. W. Nicol, Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies, Global Change Biology, 26 (2020) 103-118. DOI: 10.1111/gcb.14877. [Back]
c A. R. Ravishankara, J. S. Daniel and R.W. Portmann, Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st Century, Science, 326 (2009) 123-125; D. J. Wuebbles, Nitrous oxide: No laughing matter, Science, 326 (2009) 56-57; Donald J. WuebblesR. W. Portmann, J. S. Daniel and A. R. Ravishankara, Stratospheric ozone depletion due to nitrous oxide: influences of other gases, Philosophical Transactions of the Royal Society B, 367 (2012) 1256-1264. [Back]
d Standard atmospheric concentrations (by volume) in sea level dry air in 1976; N2 0.78084; O2 0.209476; Ar 93.4 ˣ 10−4; CO2 3.14 ˣ 10−4; Ne 18.18 ˣ 10−6; He 5.24 ˣ 10−6; CH4 1.5 ˣ 10−6; Kr 1.14 ˣ 10−6; H2 0.5 ˣ 10−6; N2O 0.27 ˣ 10−6; Xe 0.087 ˣ 10−6; O3 0.04 ˣ 10−6; NH3 4 ˣ 10−9; NO2 1 ˣ 10−9; SO2 1 ˣ 10−9; NO 0.5 ˣ 10−9; CO 0.19 ˣ 10−6; H2S 0.05 ˣ 10−9. US Strandard Atmosphere 1976, National oceanic and atmospheric administration, United States Air Force, NOAA-S/T 76-1562. Toxic carbonyl sulfide (O=C=S) also occurs to about 0.5 ˣ 10−7 % from the oceans, volcanoes, and deep-sea vents. Radon occurs in the atmosphere at about 6.0 ˣ 10−18 molar percent, produced by the decay of radium, thorium, and uranium. [Back]
e M. Appl, Ammonia, 2. Production processes, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Vol. 3, (2012) pp 139-224, DOI: 10.1002/14356007.o02_o11. [Back]
f M. Appl, Ammonia, 1. Introduction, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Vol. 3, (2012) pp 107-137, DOI: 10.1002/14356007.a02_143.pub3. [Back]
g M. Thiemann, E. Scheibler and K. W. Wiegand, Nitric Acid, Nitrous Acid, and Nitrogen Oxides, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Vol. 24, (2012) pp 177-225, DOI: 10.1002/14356007.a17_293. [Back]
h E. Culotta and D. E. Koshland Jr., NO News is good news, Science, 258 (1992) 1862-1865; S. Habib and A. Ali, Biochemistry of nitric oxide, Indian Journal of Clinical Biochemistry, 26 (2011) 3-17, doi 10.1007/s12291-011-0108-4. [Back]
i R. F. Weiss and B. A. Price, Nitrous oxide solubility in water and seawater, Marine Chemistry, 8 (1980) 347-359. [Back]
j The German Wilhelm Ostwald (1853-1932) developed this process, in 1902, for the production of nitric acid, making use of the contemporary Haber process that allowed the inexpensive production of ammonia. He was awarded the Nobel prize for Chemistry in 1909. [Back]
k The German Fritz Haber (1868-1934) developed this inexpensive process for the production of ammonia which allowed the development of the Ostwald process to synthesize nitric acid. He was awarded the Nobel prize for Chemistry in 1918. He helped weaponize chlorine during World War I. [Back]
[Back to Top ![]() ]
]
Home | Site Index| Aqueous H2S | Aqueous hydrogen halides | Aqueous biphasic systems | Aqueous phosphate | LSBU | Top
This page was established in 2019 and last updated by Martin Chaplin on 18 August, 2022