
C60 fullerene electron density

Fullerenes like C60 and C70 have remarkable hydration properties
Fullerene structures are allotropic forms of carbon, comprising an even number of sp2 hybridized carbon atoms. They form hydrophobic closed single-shelled cages. The C60 molecule is a truncated icosahedron, with 60 vertices, 90 bonds (60 between neighboring pentagonal and hexagonal faces surrounding the 12 pentagonal faces and 30 between pairs of neighboring hexagonal faces three shared around each of the 20 hexagonal faces), and 32 faces, 12 of which are pentagonal and 20 hexagonal (like a football/soccer ball). This C60 fullerene has electron-poor pentagons with long bond lengths (60 single Kekulé bonds, 0.1455 nm) joined by electron-rich shorter bonds (30 double Kekulé bonds, 0.1391 nm). Fullerene hydration is an exciting but lesser-known fact concerning the C60 and C70 fullerenes. Contrary to expectations due to their apparently hydrophobic character, they may be dissolved in water. Fullerene (C60-Ih)[5,6] can be dispersed in water (> 2 mM [303]) by transferring from an organic solvent using sonication without the need of stabilizers (for example, γ-cyclodextrin [944]) or chemical modification [271] (usually its solubility direct from solid is eleven orders lower at about 20 fM). d
Graphene
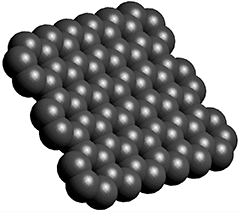
The positive interactions with water are less surprising in the light of the now-known hydrophilicity of clean young graphene surfaces (see left), where any found hydrophobicity is due to the accumulation of surface hydrocarbon impurities. [2958], which cannot easily occur on the tight spherical surfaces of these fullerenes.
It has been found that there are exceptionally strong fullerene-water interactions and rearrangement in the second-, and higher-, level hydration shells, overbalancing the expected hydrophobic fullerene-fullerene interactions [2122]. C60 fullerene has also been solubilized by ultrasonication in strong aqueous inorganic acids [579], as has C70 [1718]. The result is a kinetically stable molecular colloidal solution also containing a variety of negatively charged clusters. d It forms a transparent orange solution with a broad absorption band (400-500 nm). This fullerene is an electronegative molecule showing some aromatic behavior in the twenty six-membered (but not the twelve five-membered) rings with the pi-orbitals electron density biased outwards [301]. c C60 has been shown to have high free energy of hydration and hence a high affinity for water [1851]. An alternative view of fullerene hydration to that given below has been proposed, involving the multiple covalent hydroxylations of C60 [2121], but the mechanism and importance of this process remain unproven. e
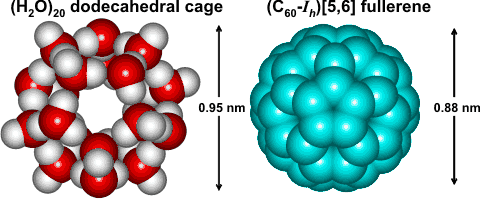 The solubility of single C60 molecules may be explained by assuming that the fullerene sits (ideally) in an icosahedral water
cluster (ES),
missing its inner water dodecahedron [1183]. The size of the C60 molecule and the central aqueous dodecahedron are similar (see opposite).
The solubility of single C60 molecules may be explained by assuming that the fullerene sits (ideally) in an icosahedral water
cluster (ES),
missing its inner water dodecahedron [1183]. The size of the C60 molecule and the central aqueous dodecahedron are similar (see opposite).
The inner twenty remaining water molecules are ideally situated to form -OH···pi hydrogen bonds a to each of the twenty six-membered rings in the fullerene, by positioning directly over these hexagonal rings; the optimum such positioning for the hydrogen bond to a benzene molecule [2534]. The twelve water pentagons possess dipoles with positive charges on the outside and negative charges on the inside, whereas the twenty water hexagons possess balancing dipoles with negative charges on the outside and positive charges on the inside; in line with the dipole on corannulene (C20H10) that has a structure similar to any of the twelve caps of C60 molecules [2982]. Thus, each of the twenty negatively-charged ends of the local dipoles interacts with a positively-charged hydrogen atom from the surrounding water. These twenty water molecules can then link through the sixty fully hydrogen-bonded water molecules from the next shell of the icosahedral (ES) cluster. This arrangement presents a negatively charged surface to the environment, as found experimentally. In such a structure, the carbon atoms are centers of electron deficiency and capable of interacting with lone pair electrons donated by extra water molecules. Such water molecules have room to sit under the outer shell water molecules to which they can hydrogen-bond if some outer shell hydrogen bonds are broken or distorted. The strong hydration around the monomeric C60 molecules has been proposed to prevent toxic reactions [687]. b
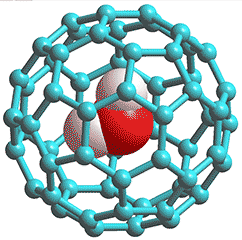
Hydrated C60 fullerene
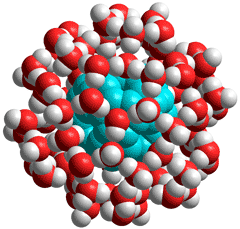
Support for this structuring has come from cross-polarization (CP) nuclear magnetic resonance experiments that have shown that liquid water within about 1 nm of C60 molecules is anisotropic and undergoes a rotational motion that is greatly hindered compared with the motion in the bulk [2110]. The surrounding water in this complex can be partially replaced by disaccharides such as lactose [1904].
The resultant symmetrical positioning of six hydroxide ions (see below right) increases the electron density so strengthening the -OH···pi-electrons hydrogen-bonding. The corresponding hydrogen ions may be associated with the water in the immediately surrounding shell or the bulk. An increased tendency to ionize by these carbon-linked water molecules would increase the negative charge on the C60 molecules, make the C60 solution acidic as found [303], and give rise to the orange color of solutions.
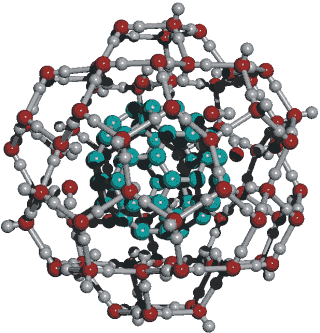
C60(OH−)6(H2O)80
Only the innermost eighty water molecules in the icosahedral cluster are shown in the structures given above, to clarify the structuring. Spectroscopic studies are consistent with this structure even though the authors propose C60(H2O)60 [602]. Interactive structures are given (Jmol). Without water hydrogen bonding to the outside of these clusters, they will collapse. The C60(H2O)60 structure has been optimized [3438] but does not have any of the twenty -OH···pi hydrogen bonds of the C60(H2O)80 structure.
Connectivity map showing potential hydroxide sites (blue)
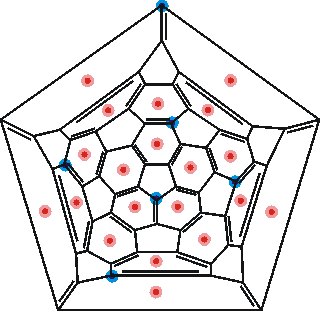
A possible arrangement of six electron-donating hydroxide ions (as shown right), shown blue, and the twenty hydrogen atom-donating hydrogen bonds, shown red, is given on the connectivity map of C60. These twenty positions are also the most prominent given by molecular dynamics simulations using a state-of-the-art quantum mechanical polarizable force field [1754].
Also shown right is the most important Kekulé structure [946a]. f
C60 molecules in aqueous solution form colloidal clusters
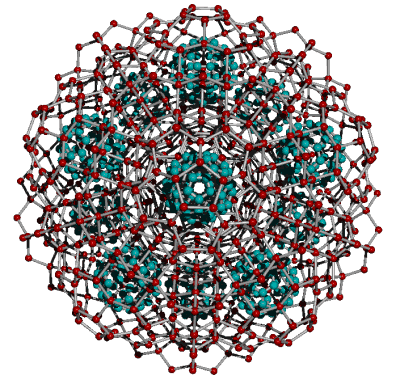
C60 molecules in aqueous solution form colloidal clusters based on 3.4 nm-sized icosahedral arrangements of thirteen C60 molecules [271], where water separates the C60 molecules [303].
The water network is formed by fully tessellated tetrahedral tricyclo decamer (H2O)10 structures (see below left for one). Such an arrangement is shown opposite within an expanded (but now strain-free) super-cluster of icosahedral water clusters. The diameter of the cluster (carbon atoms) opposite is slightly larger at 3.5 nm. There are many clusters of this size (3 nm - 4 nm) in solution (5 ˣ 108 per single 120 nm particle) [3433].
Ions that destroy the expanded water network also coagulate such C60 hydrosols (see the Hofmeister series) [302].
Tetrahedral tricyclo decamer (H2O)10 structure
connecting 4 C60 molecules
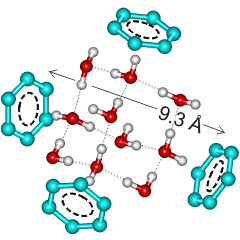
The structure is also compatible with findings by Grigoriy Andrievsky using piezo-gravimetry [303] (20 - 24 H2O per C60). This result agrees with low-temperature differential calorimetry [305] where two types of water were evident, fully hydrogen-bonded water melting at 0 °C (≈ 60 H2O per C60) showing required hydrogen-bonding to hydrogen-bond deficient water, melting at -2.3 °C (19 ± 1 H2O per C60) with 30% less enthalpy change. The ratio of the inner sphere water molecules to the outer (second) sphere water molecules varies between 1:3 for single molecules and 2:3 for infinite-sized aqueous C60 clusters. Interactive structures are given (Jmol).
Even larger clusters form up to about 80 nm diameter, but with water-mediated
···water···C60···water···C60···water····C60···water
interactions rather than hydrophobic clustering
C60····C60····C60····C60
due to the substantial C60···water interactions [2122].
Theoretical calculations show that a water molecule may be placed inside the (C60-Ih)[5,6] fullerene cage with stabilization (relative to the isolated molecule) due to stronger -OH···π hydrogen-bonding than found in the hydration sphere surrounding the fullerene [809]. Although there is room for more water molecules inside the cage, positioning them inside is energetically unfavorable due to weak hydrogen-bonding caused by the cramped environment [809].
(C70-D5h(6))[5,6] fullerene can also be dissolved in water [1146]. A solution is achieved using a mixture of sulfuric and nitric acids with or without ultrasonic irradiation. The soluble product proved to be unchanged C70 by mass spectrometry. The hydration of C70 may be similar to that of C60 as above, involving aqueous icosahedral clusters and utilizing -OH···pi-electrons hydrogen bonds a. In this case, the icosahedral water cluster (ES), again missing its inner water dodecahedron, is split into two halves hydrating the top and bottom parts of the C70 (see below). An incomplete hydration shell may form around the middle of the molecule, which naturally involves five water molecules (asterisked below) that are ideally placed to hydrogen bond to the five extra π-centers in the extra five six-membered rings that C70 possesses over the 20 in C60.
Inner sphere C70, hydrated by water cluster
C70 hydrated by water cluster
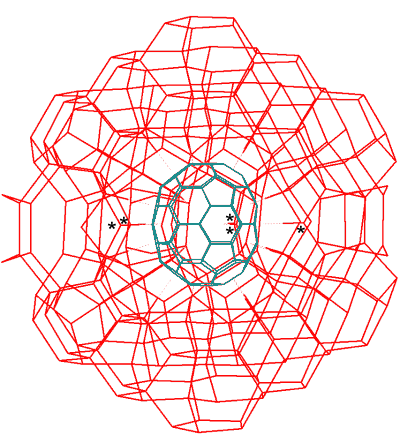
Inner aqueous sphere only, C70(H2O)90; C70 shown as blue-green, -OH···pi-electrons hydrogen bonds shown as violet-dashed with hydrogen bonds between the water molecules shown as red links, hydrogen atoms are not shown.
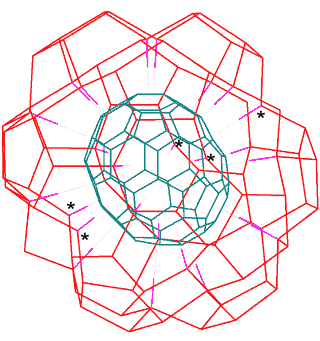
Connectivity map of C70
showing H-bonding sites to a water cluster
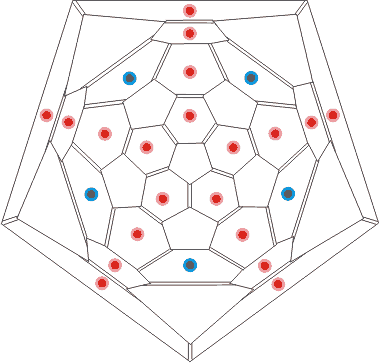
The connectivity map (Schlegel diagram) for (C70-D5h(6))[5,6] fullerene is shown opposite, indicating the electron-rich centers that may hydrogen-bond to water in the surrounding aqueous cluster. The blue dots show the positions of the additional sites (compared with (C60-Ih)[5,6]) for hydrogen bonding. It is clear that the -OH···π hydrogen bonds are not equivalent in C70 whereas they were in C60 as there are now three different environments for the electron-rich six-membered rings (in the ratio of 10:10:5).
An interactive structure of C70(H2O)90 is given (Jmol).
[Back to Top ![]() ]
]
C70 fullerene containing a water dimer [2517]
![C70 fullerene containing a water dimer [2517] C70 fullerene containing a water dimer [2517]](images/h2oc70.gif)
C60 fullerene containing a water monomer
With skill and persistence, water molecules can be placed inside fullerenes, dependent on their contained void volume; for example, C60H2O (left), C70(H2O)2 (right) [2516, 2517], and C84(H2O)3.
The properties of these contained and isolated water molecules are very different from water molecules in bulk phases of water (e.g., [2548]). Encapsulation of H2O causes a slight decrease in the electronic conductivity [3614]. Quantum calculations on the energy levels of the entrapped H2O have been reported [4353].
The energy of the ground-state encapsulated ortho-H2O are split into three levels, as are the first rotationally excited states of the encapsulated ortho-H2O and para-H2O [3510] (see also [3530] ).
[Back to Top ![]() ]
]
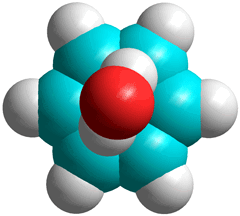
Optimized (ab initio 6-31**) positioning of
a single H2O with benzene
b Although disputed from some quarters, the toxicity widely reported for some C60 preparations may be solely due to the presence of significant amounts of solvent or impurities [687]. Tests for physiological harm should be experimental as modeling studies may ignore this hydration effect (for example, [956]). Other work has shown that hydrated fullerene may have some health benefits [1317] with antioxidant and radioprotective properties capable of reducing the harmful effects of ionizing radiation [3433]. [Back]
c Although C60 has been modeled (by some) as a purely hydrophobic structure possessing no electrostatic interactions, it is essential to recognize that the C60 molecules are polarizable and there are effective charge separations between the (positive) carbon atoms and the (negative) centers of the six-membered rings. Without allowing for these factors, such modeling is likely to be unreliable. [Back]
C60(OH)24
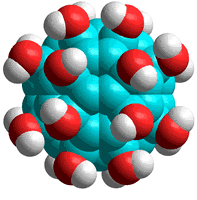
d Other work only produces colloidal clusters [1370]. [Back]
e A derivative of the C60 fullerene, C60(OH)24 (see right), dissolves and hydrates well in water. In this molecule, the hydroxyl groups are closely arranged and encourage high-density water forming around the fullerene, with limited (but strong) hydrogen-bonding to the hydroxyl groups (less than two per OH group compared with the preferred hydration of three per OH group). Consequentially, there are many broken hydrogen bonds in the surrounding water [1370]. It has been proposed that this structure may be formed during extensive ultrasonication and is responsible for the solubility of C60 [2121]. However, other studies (see above [303]) conclude that the addition of hydroxyl groups is not necessary for the solubility of the C60 fullerene. [Back]
f The 30 double bonds have bond orders of 1.44, and the 60 single bonds (all 12 pentagons) have bond orders of 1.28, so using the 'spare' Pz electrons donated from each of the 60 carbon atoms [946b]. [Back]
Home | Site Index | Novel fulleranes | LSBU | Top
This page was established in 2001 and last updated by Martin Chaplin on 1 October, 2021