
Plato thought an icosahedron could represent water.
So do I, as this theory is a useful predictor. So read on and decide if we may be correct.
Water cluster and icosahedron
mouse over
![]() Water clustering
Water clustering
![]() Cluster lifetimes and hydrogen-bond lifetimes are independent
Cluster lifetimes and hydrogen-bond lifetimes are independent
![]() Cluster equilibria
Cluster equilibria
![]() Icosahedral water clusters
Icosahedral water clusters
'...we may ask why all trees and bushes - or at least most of them - unfold a flower in a five-sided pattern, with five petals.... Some botanist might well examine the sap of plants to see if any difference there corresponds to the shapes of their flowers.'
Johannes Keplar (1611) [366]
Life on Earth depends on the unusual structure and anomalous nature of liquid water. Organisms consist mainly of liquid water. This water performs many functions, and it can never be considered merely as an inert diluent; it transports, lubricates, reacts, stabilizes, signals, structures, and partitions. The living world should be thought of as an equal partnership between biological molecules and water. Both short-range (< 1nm) and long-range (> 100 nm) [2268] organization of the water molecules has been detected.
Despite much work, many of the properties of water are puzzling. Enlightenment comes from an understanding that water molecules form an infinite dynamic hydrogen-bonded network with localized and structured clustering [1866]. The middling strength of the connecting hydrogen bonds seems ideally suited to life processes, being easily formed but not too difficult to break. An important concept, often overlooked, is that although liquid water appears homogeneous at macroscopic length scales and time scales, it is not homogeneous at the nanoscopic time or length scales (e.g., see [993]). Two processes drive the structuring of liquid water; an enthalpic process that maximizes the number and strength of the hydrogen bonds towards an optimum of four strong bonds on each water molecule, and an entropic process that maximizes the rotational freedom and translational independence of the water molecules. Working at a picosecond timescale, these two processes compete against each other, dependent on the temperature and pressure. A greater number of strong hydrogen bonds reduce the entropy and a greater freedom of movement (entropy) reduces the number and strength (enthalpy) of the hydrogen bonds. These processes are finely balanced, and there are many ways by which the same minimum energy may be achieved.
Two water tetramer clusters forming an octamer cluster
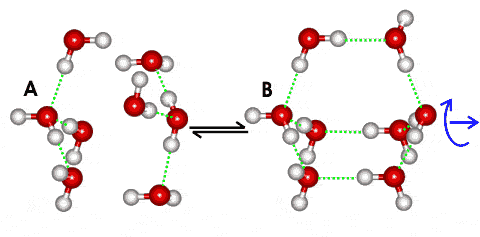
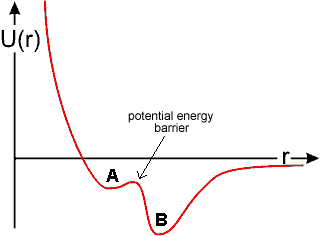
Small clusters of four water molecules (A) may come together to form water bicyclo-octamers (B) with higher negative enthalpy (more strong bonds) but lower entropy (more ordered). (A) occurs in high-density ice-seven whereas, with 60° relative twist, (B) is found in low-density hexagonal ice; (see the animated gif, 129 kB). Structures similar to A have higher numbers of 3-hydrogen-bonded and 5-coordinated water molecules as found at warmer temperatures in liquid water. In contrast, structures similar to B have higher numbers of 4-hydrogen-bonded and 4-coordinated water molecules as found at colder temperatures in liquid water [1773]. Such equilibria are balanced due to the existence of two minima in the potential energy (U) - molecular separation (r) diagram below, which shows the approach of the water tetramers. High energy x-ray diffraction experiments show that the number of water nearest neighbors does not vary over the range 254.2 – 365.9 K, with neighboring molecules switching between strong hydrogen-bonded and weak or non-hydrogen-bonded [2273], in agreement with this model.
Potential energy diagram of the approach of water
tetramers showing a shallow minimum inside
a deeper minimum; figure inspired by [16]
This competition between maximizing van-der Waals interactions (A, generating higher orientation entropy, higher density, and individually weaker but more numerous water-water binding energies) and maximizing hydrogen bonding (B, yielding more ordered structuring, lower density, and fewer but stronger water-water binding energies) is finely balanced, and easily shifted with changed physical conditions, solutes, and surfaces. The potential energy barrier between these states (see below left) ensures that water molecules prefer either structure A or B with little time spent on intermediate structures. An individual water molecule may be in state A concerning some neighbors while being in state B concerning others (for example, ice-seven). It is expected that higher-density clusters will exhibit faster water dynamics.
Indeed, simulations using ab initio van der Waals interactions support this mechanism for the density fluctuations in liquid water [1756].
The shallow minimum (A), due to nonbonded interactions, lies up to 20% inside the deeper minimum (B) due to hydrogen bonding (even allowing for a 15% closer approach of individual hydrogen-bonded water molecules). In spatial terms, minimum (A) is far more extensive as the hydrogen-bonded minimum (B) is restricted in its geometry, being highly directional. At lower temperatures (particularly below the temperature of maximum density) and pressures, the less dense structure with more extensive hydrogen-bonding at the lower minimum (B) will be preferred even though it involves a more ordered (lower entropy) structure. At higher temperatures, nonbonded interactions dominate, causing breakdown of the clustering (Figure inspired by [16]).
Bicyclo-octamer, (H2O)8
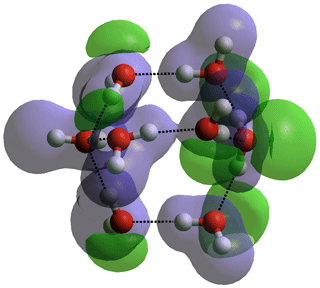
The hydrogen bonding, although cohesive, is thus holding the water molecules apart. It is the conflict between these two effects and how it varies with conditions, which endows water with many unusual properties.
These bicyclo-octamers may cluster further, with only themselves, to form highly symmetric 280-molecule icosahedral water clusters that can interlink and tessellate throughout space. A mixture of cyclic water pentamers and tricyclo-decamers can bring about the same resultant clustering. The bicyclo-octamers may also form hexagonal ice.
As all three of these small clusters are relatively stable quasi-polyhedra found, their interaction will likely produce these larger icosahedral clusters (and the latter two are the lowest energy structures out of those found by simulating supercooled water [2558]). Such clusters can dynamically form a continuous network of both open, low-density, and condensed structures.
![water pentamer, bicyclo[2.2.2]octamer and tricyclo[3.3.1.1]decamer water pentamer, bicyclo[2.2.2]octamer and tricyclo[3.3.1.1]decamer](images/water_cluster_3.gif)
Cyclic pentamer
Bicyclo-octamer
Tricyclo-decamer c
It is essential to recognize that whenever there is a cluster of water molecules in liquid water, there will be many 'decorating' water molecules on their periphery. Thus the linear H2O chain, cyclic pentamer, bicyclo-octamer, tricyclo-decamer, (H2O)20 dodecahedron, (H2O)100 (from ES),
[Back to Top ![]() ]
]
Shows how cluster lifetime is independent
of hydrogen bond lifetime (wait)
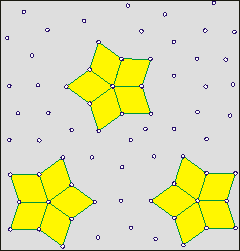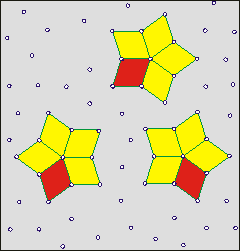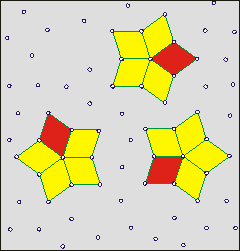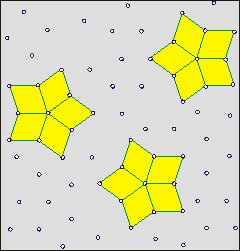
On the right is a cartoon to aid the understanding of how the lifetimes of clusters are independent of the lifetime of individual linkages. The clusters have a finite lifetime but are constantly present in the water. The cartoon shows a two-dimensional representation of a three-dimensional phenomenon. The actual clusters of water molecules are not represented. It is supposed (opposite) that the star clusters (shown yellow filled) may reform around key structures (shown as rhombuses, sometimes red, but closed ring oligomers of H2O in water). A few units move to break up the existing cluster and help create a new cluster. The new clusters are identical to the old ones but only contain a proportion of the units. Thus, dynamic clusters may reform around any of the star arms. One mechanism for such cluster reorientation involves a proton cascade leading to cluster reorientation [2423]. The movement of such clusters may involve them traveling as a wave through the medium and flickering on and off unpredictably. b
Clusters give density fluctuations at time scales below a nanosecond but water appears homogeneous at more than a nanosecond time scale [3827]. It has been shown that their lifetimes and sizes increase with decreasing temperature, particularly in the supercooled state. The interaction energy of 80% of the H-bonding molecular pairs lies between -28 and -16 kJ ˣ mol−1. The number of such bonds does not exceed 15% up to 293 K, and decreases with decreasing temperature.
Although the hydrogen bonds between water molecules in ice have equally short lifetimes to those in water, ice cubes, which can be considered as enormous ice clusters, can last forever at 0 °C in water. As there is a shift of the water O-H band center of Raman scattering (at about 3300 cm−1) to higher frequencies (shorter wavenumbers, at about 40 cm−1) with decreasing the probe pulse duration from 20 ns to ∼50 ps [3363], it is indicated that there is an increased time required for the formation of large clusters in water via hydrogen-bonding between Н2О molecules.
Similarly, but on a macro-scale on the addition of solutes, water retains its integrity as liquid water with the hydrogen bond network connected throughout the entire bulk (percolating). The local hydrogen bonds are known to be fleetingly breaking and forming [2211]. Cluster formation and lifetime are both increased by the highly cooperative nature of hydrogen bonding. Whenever low-density clusters form, they are surrounded by about the same number of 'decorating' water molecules with intermediate density.
There is a similarity, in principle, with John Conway's game "life" b in the persistence of some of its structures [1609]. [Back to Top ![]() ]
]
Cluster equilibrium, showing how the expanded low-density icosahedral cluster (H2O)280
undergoes a partial collapse to give a condensed structure followed by a further fast collapse
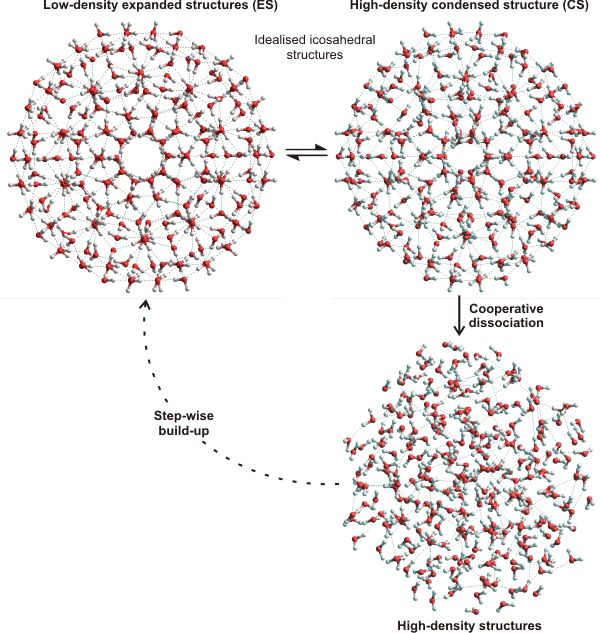
Such a dynamic fluctuating self-replicating network of water molecules, with localized and overlapping icosahedral symmetry, was first proposed to exist in liquid water in 1998 [55]. The structure was subsequently independently found by X-ray diffraction, in water nanodrops in 2001 [417]. The clusters formed can interconvert between lower and higher density forms by bending, but not breaking, some hydrogen bonds. Due to the cooperative strength of the hydrogen bonds, the structuring of the expanded structures possess slower dynamics and lower entropy whereas the high density structures possess rapid dynamics and greater entropy. Structuring may also flicker between statistically and topographically equivalent clusters but involving different molecules by shifting their cluster centers. These polyhedral structures are idealized in the diagrams but are considerably distorted and fragmented by thermal effects in reality. The existence of long-lived ring fragments is nevertheless considered to be well-founded [2053]. The cluster size required for ice formation has been estimated at about 400 molecules (one further layer of water around the icosahedral water core). However, the structure of this core structure was indeterminable [2088]. As the temperature increases the average cluster size, the cluster integrity, and the proportion in the low-density form all decrease. This structuring accommodates explanation of many of the anomalous properties of water, including its temperature-density and pressure-viscosity behavior, the radial distribution pattern, the presence of both cyclic pentamers and hexamers, the change in properties on supercooling a and the solvation and hydration properties of ions, hydrophobic molecules, carbohydrates, and macromolecules. The model described here offers a "two-state" structural model on which large molecules can be mapped to provide insights into their interactions.
The open low-density cluster ES is seen as the extreme form of water cluster with its subclusters building up. It is primarily formed in deeply supercooled water or by incorporating stabilizing solutes. It is a relatively long-lived cluster both as a whole and in its parts, particularly the (H2O)20 and (H2O)100 subclusters. Its collapse into the condensed structure (CS) is expected to be a significant route for its degradation (rather than the slow loss from its exterior) that may be caused by increased pressure, temperature, or destabilizing solutes. This sudden extensive and comprehensive collapse is a crucial feature of the two-state structuring of water. The condensed structure (CS) is far less stable than the ES structure or its substructures. It can further change either by reforming ES or by a further abrupt and comprehensive collapse to form high-density stranded and small ring structures.
[Back to Top ![]() ]
]
a As the temperature of supercooled water drops further below 0 °C, the density, self-diffusion, thermal conductivity, enthalpy, and entropy all decrease, whereas compressibility, viscosity, thermal convection, specific heat (CP), and gas solubility all increase. As the pressure increases on supercooled water, viscosity and freezing point decrease, whereas entropy and self-diffusion increase. [Back]
This image was made using Life32 v2.15 beta,
by Johan G. Bontes
b The Game of Life is 'played' on an infinite two-dimensional grid of squares, each of which is in one of two possible states, alive or dead (see right for an example). Every cell interacts with its eight adjacent neighbors using the four rules:
The Game of Life shows how a 'cluster' can last longer than its components. [Back]
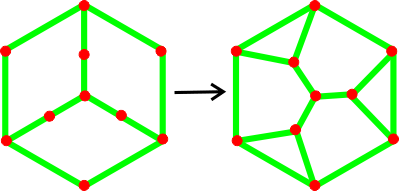
The 335361 water decamer derived from the
low-density (H2O)10 tricyclo-decamer
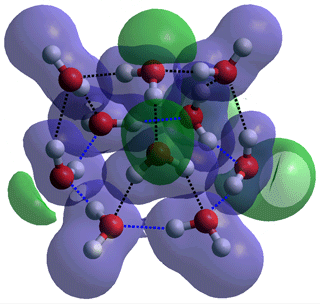
c Counter-intuitively, the 64 structure of the tricyclo-decamer (H2O)10 degrades into a 335361 structure with a flattened hexamer ring (see right, connected by blue dotted lines) on optimizing its structure using the ab initio 6-31G** calculations. This is due to the lack of outer hydrogen bonding from water molecules exterior to the cluster and is an example of the collapse from low-density structures to high-density structures where no stabilizing factors are present. It also shows the tendency for isolated water clusters to maximize the number of hydrogen bonds over their individual strengths.
Connectivity maps showing the change in hydrogen bonding
 [Back]
[Back]
![]() Introduction to water clustering
Introduction to water clustering
![]() The icosahedral water cluster, (H2O)280
The icosahedral water cluster, (H2O)280
![]() Evidence for the icosahedral cluster model
Evidence for the icosahedral cluster model
![]() Conclusions concerning water clustering
Conclusions concerning water clustering
Water: Home| Methods for investigating clusters | Brief history of water clusters | LSBU | Top
This page was established in 2000 and last updated by Martin Chaplin on 17 September, 2021