
Water molecules in a (6; 6) carbon
nanotube, from [4148]
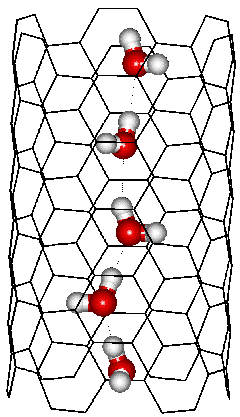
This diagram shows the most stable chain of water molecules (a one-dimensional strand of water molecules), each with two hydrogen bonds, within a (6,6) carbon nanotube (CNT) of 4.12 Å in radius and 13.72 Å in length immersed in water. The water self-aligns to give an almost all-trans zigzag arrangement with similar entropy found in bulk water [1397]. Each water molecule has two cooperative hydrogen bonds plus multiple van der Waals interactions from the carbon nanotube. Such organized water chains are determined by the confinement rather than interaction with the pore surface [1398] and make rapid proton conductors due to the Grotthuss effect [1041].
Confined water is generally liquid water held within nanometer-sized vessels.
![]() Capillary rise
Capillary rise
![]() Venturi effect
Venturi effect
![]() Interfacial water and water-gas interfaces
Interfacial water and water-gas interfaces
![]() Nanobubbles
Nanobubbles
![]() Hydrophobic confinement
Hydrophobic confinement
![]() Hydrophilic confinement
Hydrophilic confinement
![]() Mixed confinement
Mixed confinement
![]() Water molecules confined in beryl nanovoids
Water molecules confined in beryl nanovoids
Confined water is found widespread in granular and porous materials and around and within cells, macromolecules, supramolecular structures, and gels. It has been reviewed [1592, c as has its flow properties [3601]. When water is confined, there is a conflict between the energetic minimization of the hydrogen-bonded network, interactions with the relatively large cavity surface, the possibility of quantum coherence (orderliness), and the fit within the space available. The physical properties and state of the contained water may vary widely depending on the molecular characteristics of the cavity surface, confinement dimensions, temperature, and pressure. The properties of the confined water are difficult to predict and may be very different from those of bulk water, exhibiting more significant anomalies. Unsurprisingly, the effect of charged surfaces, such as negatively charged reverse micelles, are much more extensive than those of hydrophobic surfaces, such as droplets in oil [2783]. This is particularly true when the confinement is on the nanoscale. When the confinement is about or less than 2 nm, the water cannot form hexagonal or cubic ice crystals such that cooled water turns into supercooled water rather than ice even through the 'no man's land' (150 K < T < 227 K).
Water confined in zwitterionic liposomes has been examined for conformation changes using second harmonic scattering. There was long-range H-bond network confinement effects in light water ( > 140 nm; > 4 ˣ 107 H2O molecules) but not in heavy water (< 58 nm; < 3 ˣ 106 D2O molecules) [4471].
The properties of deeply supercooled liquid are often used as indicative of the properties of liquid water in no man's land. It is best to take care over such conclusions as the confined water properties can vary widely with its position even within the same confinement. This scenario has been carefully examined [2613], but without concluding that such confined supercooled water is likely to be identical to a continuation of bulk supercooled water. This is unsurprising as the space available for the molecular clusters (=< 2 nm) is insufficient for the expected structure of the low-density form of water to be formed, particularly if the further space reduction due to surface interactions are taken into account. Proton-charge mobility has been investigated in nanoscopic water droplets [3187]. It slows down significantly with decreasing droplet diameter below 5 nm, with the diffusion constant being about 100 times smaller than in bulk water for diameters <1 nm. This probably results from the more rigid hydrogen-bond network in this nanoconfined water. Trace amounts of water, confined in inter-nanoparticle gaps (nanomenisci), show ice-VII-like characteristics [3525].
[Back to Top ![]() ]
]
Water at hydrophobic surfaces is similar to that at the liquid-gas interface. However, confinement prevents movement of the water molecules towards the surface, and van der Waals dispersion interactions loosely hold the outer molecules in preferred positions and orientations relative to the surface atoms.
Much theoretical and experimental work has concerned water in carbon nanotubes [3431], made up of a hexagonal lattice of sp2-hybridized carbon atoms rolled up into cylinders with diameters of about a nanometer. Such structures are often thought of as hydrophobic cylinders, but that can underestimate the importance of the surface. The carbon atoms provide many van der Waals interactions in an orderly hexagonal lattice arrangement that varies between the different nanotube types and their diameters. Although uncharged, these surfaces provide extensive pi-electron orbitals that can interact with and be polarized by, the water molecules to cause effects significantly different from that expected of a flat and uniform hydrophobic cylinder. The preferred arrangement of the water molecules within these nanotubes is dependent on their diameter and the temperature and pressure.
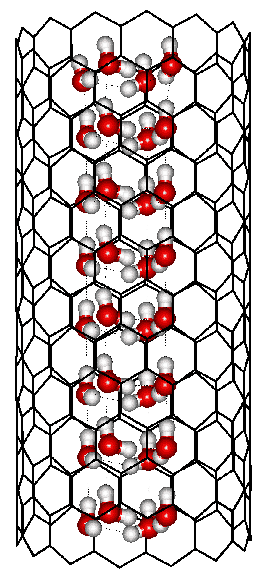
Square ice formed inside a (14; 0) carbon nanotube
In modeling studies, the penetration of water into nanotubes (see also top right) is modulated by small changes in the polarity of the wall and the dimensions of the cavity [1396]. The cohesive nature of the hydrogen-bonded network may allow water to travel quickly through such pores, with the molecules leaving the pore pulling through a following stream of water. Thus, sub-micrometer-thick membranes made from graphene oxide (a mixed hydrophilic/hydrophobic material), which are impermeable to all other liquids, vapors, and gases, allow unimpeded permeation of water [1778]. Graphene sheets with nanoscale pores also function well as desalination membranes [1900].
A phase diagram of water within carbon nanotubes has been proposed for tube diameters up to 1.7 nm. This suggests that there may be at least nine phases possible within the cylindrical space, including those found by x-ray diffraction and by simulation. The global maximum freezing point is found to be for ‘square’ ice at 290 K [1400].
Shown opposite is such a square ice nanotube formed within a (14; 0) carbon nanotube of diameter 1.11 nm.a Each water molecule has four cooperative hydrogen bonds, which are more bent than in hexagonal ice. They also have multiple van der Waals interactions from the carbon atoms.
Other ices (for example, pentagonal, hexagonal, and heptagonal nanotubes of water molecules, with two donor and two acceptor hydrogen bonds) are formed within carbon nanotubes of diameters increasing up to 1.9 nm diameter [1401]. Conversion between these ices can be achieved by increasing the pressure, such that the square ice converts to the pentagonal ice at about 200 MPa at about 275 K [1402]. The density and self-diffusion coefficients of liquid water in narrow confining nanotubes generally increase with their diameter but exhibit anomalous behavior in (8; 8) and (9; 9) carbon nanotubes [2292]. Water at low density can have a lower diffusion inside nanotubes than water at higher densities [3431b].
Interestingly, ambient water flow through carbon nanotubes with diameters of less than 2 nm exceeds predictions by more than three orders of magnitude and is substantially higher than using larger diameter pores [2345]. This is probably due to a nearly frictionless surface and the confined space preferring water chains rather than 3-D clusters, reducing the drag. Single-file water flows down 0.8 nm diameter carbon nanotubes an order of magnitude faster than within biological water transporters [3015]. There is an anomalous flow of ice-like water through carbon nanotubes (diameter 1.17 nm) at room temperature similar to that otherwise only occurring in superfluid helium. It requires a slight difference in temperature between the ends of the tube. The ice-like water moves collectively and converts the thermal energy spontaneously into the directed motion without any energy loss. A pressure difference of up to 25.6 MPa may be generated from a temperature difference of just 23 K [3054].
Molecular dynamics calculations have shown that the static dielectric constant of water in carbon nanotubes may increase 10-fold to 700 due to the surface excluded volume allowing an increase in dipolar fluctuations [2372]. The presence of this expected layer of “free” OH bonds facing the nanotube inner wall has been found experimentally for liquid water in larger diameter (1.4 - 2.1 nm) nanotubes [2777]. However, it has also been reported from capacitance measurements that there is an anomalously low dielectric constant of graphite and hexagonal boron nitride confined water, down to about 2.0 for water confined to about 2.0 nm [3319]. There are orientationally ordered surface water molecules that show reduced fluctuations in their collective dipole moment [4180]. However, these are active at a molecular level, with finite residence times, it displays time scales similar to more distant water layers. Water further from these surfaces shows low 'averaged' dielectric due to the presence of this surface water.
The phase boundaries of water confined within carbon nanotubes show substantial sensitivity to the diameter [2875]. Ice was formed, melting between 105-151 °C (pore diameter 1.05 nm), 87-117 °C (1.06 nm), -35 and 10 °C (1.15 nm), < -80 °C (1.24) 15–49 °C(1.44) and 3–30 °C (1.52 nm). Both low- and high-density liquid water states are detected near ambient temperature and above ambient pressure within 1.25 nm carbon nanotubes. In the temperature-pressure phase diagram, the low- and high-density liquid phases are separated by the hexagonal ice nanotube phase [2925]. Many other phase diagrams for water, confined in graphene nanocapillaries and single-walled carbon nanotubes, have been proposed [3234], using molecular dynamics simulations.
Square ice formed between graphene sheets,
from [2312]
![square ice formed between between graphene sheets, [2312] square ice formed between between graphene sheets, [2312]](images/square-ice.gif)
The graphene-water interface has been probed by surface-sensitive infrared-visible sum-frequency generation (SFG) spectroscopy that gave a strong SFG peak associated with highly coordinated, ordered water next to the graphene [2935]. A slightly irregular two-dimensional square ice ([2312], see left) forms between sheets of graphene 0.65 nm apart under ambient conditions. It has a high packing density and is thought to exist under nano-confinement by many hydrophobic surfaces. This square ice is not the only two-dimensional ice possible as confined water, under pressure between flat plates, 0.85 nm apart, forms a lower density twelve-fold symmetrical quasicrystal made up of mostly five-membered rings in two molecular layers [1705]. Three different ice structures were found between 0.8 and 1.0 nm spaced hydrophobic walls. One of these was an interlocked pentagonal bilayer ice (IPBI), with (per unit cell) six pentagons (including those sharing vertices) along the x-direction and two pentagons (without shared vertices) along the y-direction [2691]. From first-principle calculations, a phase diagram of double-layer ice as a function of pressure (0.1 MPa -5 GPa) and confinement (0.85 - 1.1 nm width) at 0 K has been predicted [2907]. Molecular dynamics simulations have been used to compare the liquids, water, argon, and a simple metallic liquid, in a graphene slit pore. With water, the monolayer minimum is entropy-driven with large diffusion oscillations, whereas the liquid argon and metal were energy-driven and entropy-driven with much smaller diffusion oscillations, respectively [4401].
Water confined between hydrophobic MoS2
and graphene, showing the oxygen atoms only
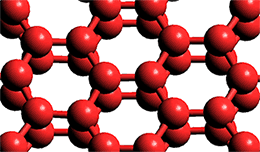
Graphene
Water confined between hydrophobic molybdenum disulfide (MoS2; a flat hexagonal sheet-like structure with sulfur-covered faces) and graphene (a flat hexagonal sheet-like sp2-hybridized carbon structure, see left) gives a flat two-layer crystalline ice phase. This phase consists of two planar hexagonal layers with water molecules directly on top of, and hydrogen-bonded to, each other (three 120° and three 90° angles between the hydrogen bonds; see right where only the oxygen atoms are shown). The intra-hydrogen-bonding between the layers gives rise to four-membered square rings [2952]. The lack of any surface dangling bonds gives rise to this ice's hydrophobic character. The surface of clean young graphene (see above left) is hydrophilic with any found hydrophobicity due to the accumulation of surface hydrocarbon impurities. [2958]. Approaching water molecules prefer to sit with their oxygen atoms pointing at the center of the carbon hexagons to maximize the van der Waals contacts between the electronic charge density of the O atom and the delocalized p-electron density of the graphene (13.5 kJ ˣ mol−1, [2978]). Similarly, water confined within narrow slits at supercooled temperatures has been found to show many 2-D phases of ice and amorphous ice [3418].
There is evidence that the protons of confined liquid water exist in a novel quantum state when significant quantum fluctuations in charge take place along the hydrogen bonds [1699].
If water is forced into crevices in a hydrophobic/super-hydrophobic surface, the high surface tension of water-hydrophobic solid interfaces stores energy in the form of interfacial free energy. The spontaneous extrusion of such water out of the nanopores, related to pressure changes, allows the partial or complete recovery of the stored energy [2701]. In these hydrophobic confined carbon nanotube systems under pressure, the transport of water molecules proceeds as a wave motion with frequencies in the terahertz region caused by the hydrogen-bonding and determined by the carbon nanotube diameter [2835].
Volumes of water confined within hydrophobic clefts and pores (see elsewhere) have higher activity than bulk water. The water tends to exit from these volumes creating 'closing' pressures. This evaporation of water induced by confinement between hydrophobic surfaces has a functional role in numerous biophysical phenomena, including hydrophobic self-assembly. Water expulsion is extremely sensitive to the flexibility of the confining material. If the material is more flexible, the pores preferably empty the liquid phase [2927]. Water bridges can form between disjointed nano-channels, such as carbon nanotubes, under an applied pressure drop, and these have been studied [4070]. They may connect separate narrow carbon nanotubes that contain single-file chains of water, dependent on the water pressure drop.
Heavy water (D2O) binds more strongly within hydrophobic pores than light water (H2O), and this may, in the future, lead to a heavy water concentration system [3000].
[Back to Top ![]() ]
]
At hydrophilic surfaces, polar and hydrogen-bonding interactions with the surface may vary between those much weaker than water-water hydrogen bonds to those much stronger [3849]. Water will condense from the gas phase onto glass to give at least four layers of absorbed water [1403], with the water next to the silica highly structured, [3211], dependent on the surface and distinguishable from more distant water by infrared [1404] X-ray [1405] spectroscopy and molecular dynamics [2854]. The negatively-charged silica surface has many isolated, vicinal, and geminal silanol groups, dependent on its history [2883], that strongly hydrogen bond to water. The pKa of the silanol groups vary between 2.3 to 10.7 as determined along the capillary wall due to surface heterogeneities [3016]. Mineral oxides, such as quartz (SiO2), sapphire (Al2O3), and hematite (Fe2O3), are generally thought to be hydrophilic and well wetted by water. However, fresh faces have a modest hydrophobicity depending on how the surfaces have been prepared. Given sufficient time and an appropriate pH, surface hydroxylation occurs, and the surfaces become hydrophilic [2858]. Even the second neighbors to the surface are considerably affected due to the competition between the surface water and the effects of confinement [1406]. When the confinement is less than about 2 nm wide, the viscosity may increase considerably to over a million times that of bulk water, but this viscosity increase is not noticed if the confining surfaces are hydrophobic [1304]. The surface hydroxyls, on the pore wall, hydrogen bond to the water and control the distortion of the hydrogen bond network and its surface dynamics [1408]. The dynamic behavior of water (models) within nanopores and biological channels in lipid bilayer membranes has been reviewed [4073].
Hysteresis in the adsorption/desorption
of water on hydrophilic surfaces
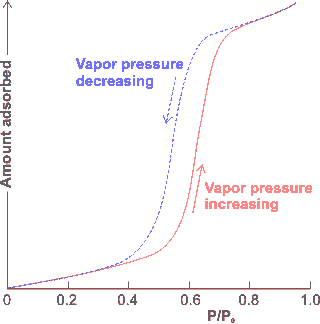
Variation of the spacing between any interaction sites on polar cavity surfaces determines the strength of binding, distribution, and organization of several layers of water molecules. Sawtooth-like oscillatory forces may be generated as hydrophilic surfaces approach with about 1 nm periodicity, corresponding to units reducing by clusters with a linear width of five hydrogen-bonded water molecules at a time (rather than single water molecule losses) [2105].
The adsorption of water to a bare surface involves continuous structuring changes due to relaxation of the hydrogen-bonding and molecular orientations as the surface becomes covered and further water layers are added. This can give rise to substantial hysteresis (shown on the right) in structure and properties on its removal [1409]. The adsorption of liquids into hydrophilic nanopores is due to both capillary flow and surface hydration, followed by the growth of the surface films. This latter mechanism can force out compressed gas from within the pores [2977].
The adsorption of water vapor into hydrophilic mesoporous TiO2 has been studied by NMR and multiscale molecular dynamics simulations [3496]. Three different water dynamics environments were distinguished and quantified: a neighboring layer up to about 0.75 nm, where strongly bound water molecules exist at the inner surfaces and effectively close the pore diameter; a second less structured layer but still with restricted mobility extending over about a nanometer; and a bulk-like fraction of mobile water that is still somewhat affected. The pore filling is modulated for periodically alternating hydrophobic-hydrophilic pore surfaces [3497].
Confinement may cause raised or lowered melting points, dependent on the surface and the space available. Generally, it might be expected that the raised boiling point and depressed freezing point are inversely related to the capillary diameter due to the Gibbs-Thomson effect [1420]. As examples, cubic ice may form in 4.2 nm diameter cylindrical silica pores at 232 K [1410] and water confined in the well-ordered, if narrow (diameter 1.5 nm), pore structure of the silica MCM-41-S-15 does not freeze above 130 K [2180]. The possible liquid-liquid coexistence line in supercooled water, below its homogeneous nucleation temperature, has been investigated [3775] in wider pore structured MCM-41/C10 (diameter 2.8 nm and total pore volume 0.5 ml/g). The temperature range from ambient down to 120 K, at pressures between 70 MPa and 300 MPa, was used. This work did not support the presence of two liquid phases but provided evidence for the coexistence of liquid water and ice. Surface interactions and the confinement diameter may cause opposite effects, such as the ice that forms at 400 K in very narrow pores (0.6-1.0 nm diameter) in porous glass [1411]. Two dynamically different fractions of confined water have been found to coexist within the pores of mesoporous silica particles with pore diameters ranging from 2.1 nm to 5 nm and pore length of about one micrometer. The two fractions bore similarities to the two ultra-viscous liquid waters (HDL, LDL) found in supercooled bulk water and were found to coexist over a range of temperatures [3573].
Water may freeze when confined between flat surfaces, so forming the optimally ordered hydrogen bonds required. The confined water undergoes significant changes in density and viscosity as the surface separation is varied. For example, in a model, water goes from liquid to solid and back again as the space, between interacting but non-hydrogen-bonding surfaces, decreases from 0.58 nm to 0.53 nm and then to 0.47 nm [1412]. If unable to form the required ordered structures, it may still undergo wide fluctuations in density as it attempts to optimize its structure within the confinement restrictions
When ice does form at strongly binding or atomically rough confining surfaces, there may be no room for expansion and a high-density amorphous ice may result [1413]. Various ices may form in interacting confined space, with a high-density confined ice (1.2 g cm−3) formed from two helices and crystallizing at ambient temperature in AlPO4-5 zeolite 0.73 nm diameter micropores [1407]. Both cubic and amorphous ices can form at strongly binding silica surfaces at low temperatures [1406]. However, rather than forming ice within confinement, liquid water in confined spaces may leave the confinement on freezing to crystallize freely external to the cavity, so long as the gas phase can easily replace them under the ambient pressure [1399]. [Back to Top
![]() ]
]
An important form of biological confinement is where there are both hydrophilic and hydrophobic effects like those existing in the binding sites of proteins. Hydrophobic pores terminated by hydrophilic binding sites have been investigated [3484]. A good guest for such molecular pores meets the following criteria: (a) the hydrophilic atoms in the guest are complementary to the hydrogen-bonding sites at the end of the cavity in terms of orientation, distance, and number; and (b) the hydrophobic group of the guest is sufficiently large to expel all of the cavity water molecules and maximize the hydrophobic effect, but not so large to introduce steric repulsion with the cavity walls.
Emerald

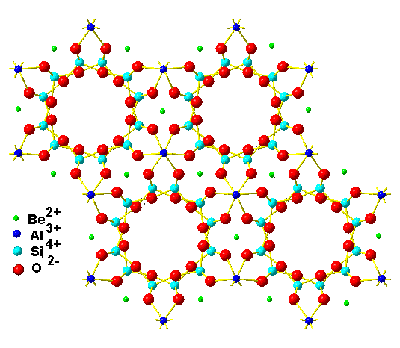
Beryl is a colorless hexagonal mineral composed of beryllium aluminum cyclosilicate with the chemical formula (BeO)3(Al2O3)(SiO2)6 that can be colored by trace amounts of other ions such as chromium (emerald, shown on the right) and ferrous (aquamarine); for structure see below right. Beryl contains SiO4 tetrahedra-lined hexagonal voids just 0.51 nm wide by 0.9 nm long, separated by narrow (0.28 nm) “bottlenecks” (Si6O6 rings) that hold single isolated water molecules.
The structure of beryl, showing the hexagonal voids
These water molecules exhibit quantum tunneling behavior whereby the water molecule hops into 6 × 2 equivalent positions above and below the oxygen atom within the channel, that spread out the positions of the hydrogen atoms (delocalization) into a pair of corrugated rings (see below with the calculated hydrogen charge density, going from blue (lowest) to yellow (highest) [2547].
The hydrogen charge density of water
molecules within the beryl voids [2547]
![Hydrogen charge density of water molecules within the beryl voids [2547] Hydrogen charge density of water molecules within the beryl voids [2547]](images/beryl3.gif)
Thus, the tunneling causes water's dipole to disappear and its center of mass to shift to the central axis (the crystalline 'c' axis) of the double ring structure [2547].
[Back to Top ![]() ]
]
a The octameric cubic structure (H2O)8 has also been found by crystallography, trapped within supramolecular metal–
organic networks [2093]. Its structure in the gas phase has been explored through terahertz spectroscopy [2595]. [Back]
Home | Site Index | Water-gas interface | Nanobubbles | LSBU | Top
This page was established in 2000 and last updated by Martin Chaplin on 18 July, 2022