
512 64 cavity
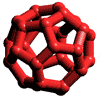
![]() Ice-seventeen (ice XVII)
Ice-seventeen (ice XVII)
![]() Other potential low-density ices
Other potential low-density ices
![]() Negative pressure
Negative pressure
If ice is stretched (i.e., put under negative pressure), a it may form (meta-) stable ultra-low-density ices. Such ices may be stable at ambient pressure (positive) in the presence of interstitial molecules that prevent structural collapse. However, under negative pressure (metastable conditions with respect to mixed gaseous water/hexagonal ice), the need for these stabilizing molecules may not be required. At present, there are three such ices; two (ice XVi and ice XVII) have been experimentally proven while the other (empty clathrate s-III), although strongly supported by modeling, has not yet been experimentally proven.
Ice-XVI (clathrate-II) crystal structure
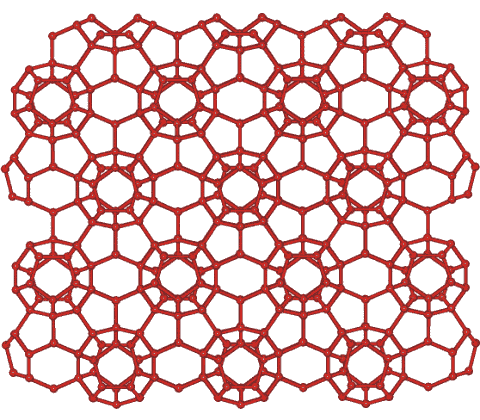
First described from theoretical considerations [3773], ice XVI may be formed from neon hydrate (a clathrate of structure CS-II) after five days of continuous vacuum pumping to remove all guest neon atoms [2252]. On removal of the neon, the hydrogen bonds elongate, and ice XVI expands, with the 512 cavities expanding by 3.9 % and the larger 51264 cavities expanding by 3.3 %, both due to the loss of the attractive van der Waals interactions. It is the least dense of all experimentally-known crystalline water phases (0.81 g.cm−3) and is expected to be the stable low-temperature phase of water at negative pressure but collapses and decomposes at 145 K. It has very slightly larger lattice constants (0.1% increase) than the filled hydrate at low temperatures [2252].
The structure of ice XVI is the same as the cage structure of CS-II clathrate hydrate and shown left (the water hydrogen atoms have been left out). Cubic crystals contain sixteen 512 cavities, eight larger 51264 cavities, and 136 H2O molecules per unit cell. The tetrahedral 51264 cavities form an open tetrahedral network, with their centers arranged reminiscent of the cubic ice structure and separated by groups of three 512 cavities.
While the thermal expansivities of the filled CS-II hydrates are positive as usual for solids, ice XVI exhibits negative thermal expansivity below 55 K as with other ices such as hexagonal ice [3029].
The hydrogen bonding is random, following the 'ice rules'.
For interactive Figures, see Jmol.
3x3 unit cells of clathrate s-III highlighting the two types of cavity
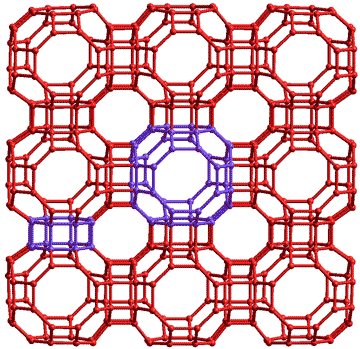
There are very many potential low-density ices that may be discovered by modeling. For example, their structures may be related to the 219 four-coordinated structures in the zeolite database [3039]. It has been found that (models of) some of these are stable near absolute zero under negative pressure and have densities approaching zero. These 'aero-ices' consist of long struts of hexagonal cross-section joined at polyhedral vertices and enclosing vast empty spaces. The fact that such structures are found to be stable by modeling does not make them more likely to be found experimentally so that they are not deserving of numbering as new ices. Some are interesting, however.
A cubic S-III clathrate (made up of two large 4126886 and six smaller 4882 cavities with 48 water molecules per unit cell, see left where one each of these novel cavities are highlighted, and the water hydrogen atoms have been left out) has been found theoretically by modeling [2507] with a lower density (0.59 g.cm−3 without interstitial molecules) than ice XVI. It is expected to be more stable than ice XVI at pressures below (more negative) than about -400 MPa at 0 °C.
The large 4126886 cavities were stabilized by the presence of dodecahedrane (C20H20) molecules.
12464418 cage showing the four 12-water rings
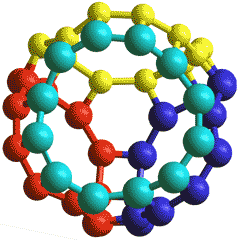
Another such low-density structure (0.506 g cm3) is a potential cubic crystalline phase of ice clathrate, which is composed of eight very large icosahexahedral cavities (12464418)(shown right; all 48 balls shown are water molecules forming four tetrahedrally positioned 12-membered rings), eight intermediate dodecahedral cavities (6646), and sixteen very small octahedral cavities (6246) per unit cell (192 H2O, Space group Fd-3) [2862]. The icosahexahedral cavities (12464418) are both strained due to the large number of four-membered rings, unstable in that they easily collapse, and highly porous due to the large ring sizes.
Using computer simulations, the phase diagram of ice under negative pressure has been constructed, including the ice polymorphs [3772].
[Back to Top ![]() ]
]
The stability limit of water at negative pressure,
![The stability limit of water at negative pressure, from [1886,4142] The stability limit of water at negative pressure, from [1886,4142]](images/speedy.gif)
a Negative pressure. Negative pressures are possible for liquids and solids but not achievable for gases [4203]. Negative pressure is a metastable state of water, with respect to vapor. Its metastability is terminated by the nucleation of a bubble. It is achieved by stretching the material under tension, which is then metastable with respect to the production of a vapor state that occurs via a nucleated process with an activation barrier. Materials at negative pressures are subject to cavitation but may exist for extended periods (years) at minus several MPa, down to a limit of about minus 10 MPa, and can remain intact for long periods until this activation barrier is overcome [3950]. The Figure on the right shows the stability limit first described by Speedy [1886]. Later work indicates that it may not stretch quite as far to the left as indicated (~-180 MPa). Several properties of water under negative pressure have been calculated using simple models (CV (max), CP (max), ΚT (max)) [2993].
Water is the most stable liquid to negative pressure due to its cohesiveness.
Practical examples are the transpiration of water from the root to the leaves in plants that is driven by a reduction of pressure in the leaves, the grip of octopus suckers, and the catapulting mechanisms of fern spores.
It should be recognized that 'negative pressure' is not 'less than zero pressure, but 'in the direction opposite to positive pressure', i.e., tension).
[Back]
Home | Site Index | Ices, introduction | Ice-Ih | Ice-Ic | Ice-Isd | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2015 and last updated by Martin Chaplin on 4 October, 2021