
Clouds over the Himalayas

Stacking-disordered ice is the natural structure of cubic ice
The solid form of water that forms in cold high clouds has been found to consist of mixed crystals of low density amorphous ice, stacking disordered ice (stacking-faulted hexagonal ice Ih) and hexagonal ice [4457]. Stacking disordered ice is a metastable form of ice intermediate between cubic and hexagonal ice. It is important to Earth’s climate and, through ice nucleation rates, to accurate weather and climate forecasting. Many early studies on cubic ice were subsequently proven to contain substantial amounts of stacking disordered ice. Ice crystals formed on homogeneous ice nucleation in deeply supercooled water nanodrops (r ≈ 10 nm) at ∼225 K were only 78 % cubic ice with 22% hexagonal ice; effectively 44% stacking-disordered ice [3032]. Dielectric and nuclear magnetic resonance investigations into warming stacking disordered ice have shown that traces of the disordered ice remain up to 270 K [3274].
Shown below is the interesting and relevant structure consisting of alternating sheets of hexagonal ice and cubic ice, which was first described on this website in 2000. This structure is now thought to play a significant part in cubic ice structures and atmospheric ice nucleation, with randomly mixed hexagonal and cubic ice planes often found [1236]. The proportion of cubic ice structure may vary, and ice nuclei can present stacking disorder in all directions. The ice is called stacking disordered ice Isd. Using modeling, stacking-disordered ice crystallites at 230 K are shown to be about 14 kJ ˣ mol−1 more stable than hexagonal ice crystallites, making their ice nucleation rates possibly more than three orders of magnitude higher than predicted by classical nucleation theory [3131]. It is found in several cloud types, including upper tropospheric cirrus clouds and contrails formed by aircraft [2304]. In the bulk phase, stacking-disordered ice is metastable with respect to hexagonal ice at most ground temperatures having a higher vapor pressure, stability being: ice Ih > ice Isd > Ice Ic.
Alternating hexagonal/cubic ice unit; two hexagonal sheets followed by a cubic sheet
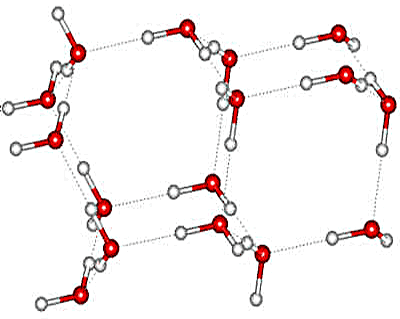
Alternative ice Ih/1c lattice
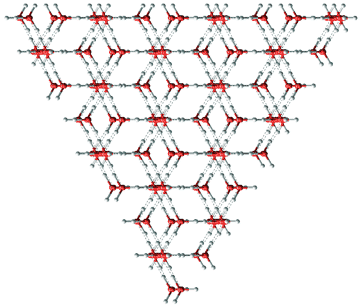
Note that in all these structural diagrams, the hydrogen-bonding is ordered whereas, if the structure were to exist, the hydrogen-bonding would in all likelihood be random (obeying the 'ice rules': two hydrogen atoms near each oxygen, one hydrogen atom on each O····O bond). Also, the stacking disorder would be more random than alternate. The (perfectly ordered) crystal structure appears to be hexagonal with unit cell (8 molecules) dimensions 4.48 Å (a,b) and 14.62 Å (c). It belongs to the trigonal P3m1 space group with threefold rotational symmetry. The form of Isd with ordered hydrogen bonding has not yet been found.
Trigonal snowflake,
from SnowCrystals.com
Stacking disordered ice gives rise to trigonal snowflakes (see above left, from SnowCrystals.com) [2304]. An estimation of the relative proportion of cubic sequences in the stacking sequences has been attempted by using neutron or X-ray diffraction patterns [3213].
X-ray diffraction; comparison of the low-pressure ices; from [2349]
![X-ray diffraction; comparison of the low pressure ices; redrawn from [2349] X-ray diffraction; comparison of the low pressure ices; redrawn from [2349]](images/stacking.gif)
Hexagonal, cubic, and stacking disordered ices are indistinguishable using vibrational spectroscopy but can be easily told apart using X-ray diffraction [2349]. The cubicity of stacking-disordered ice samples can be determined from experimental distribution functions [3392]. The calculated X-ray diffractograms of cubic and hexagonal ices are shown right with their Miller indices (lattice planes) together with the powder X-ray diffractogram of a stacking disordered ice [2349].
Ice crystals giving miller indices
(x,y,z) of faces; from [2304]
![ice crystals giving miller indices (x,y,z) of faces; from [2304] ice crystals giving miller indices (x,y,z) of faces; from [2304]](images/ice-miller.gif)
The (001) plane of ice Ih and the (111) plane of ice Ic are identical to the (001) plane of ice Isd, allowing alternating cubic and hexagonal layers to stack. Ice Isd does not necessarily consist of alternate single sheets of hexagonal ice and cubic ice but may usually be found with one or several sheets of hexagonal ice followed by one or several sheets of cubic ice in an apparently random sequence dependent on the freezing history of the ice [1236e]. The difference in energy between ice Isd and ice Ih of the crystals has been estimated variously as 13 to 160 J mol−1 [2559] depending mainly on the degree of cubicity of the ice Isd.
Similar hexagonal, cubic, and stacking disordered crystal types have been found in diamond, which is isostructural with ice I [2392]. They have also been found in isostructural AgI [3506] where fine particles act as nuclei in cloud seeding [3738].
Interactive structures are given (Jmol).
Interestingly, stacking-disordered diamond has also been found with both cubic and hexagonal stacking motifs [3657]. Also, similarly disordered structures have been found for solid ammonium fluoride (NH4 F) [4432].
[Back to Top ![]() ]
]
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Ic| II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | XVI| XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2001 and last updated by Martin Chaplin on 16 June, 2022