
Face-centered cubic oxygen lattice
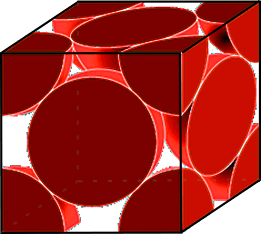
The state of ice at the very high pressures above ice X has only recently been reached experimentally. Modeling gives a confusion of possibilities. As such modeling, but of lower pressure ices, does not provide accurate results compared with experimental structure information, it is expected that these results are indicative at best. Density functional calculations [1709] indicate a pressure-induced initial displacement of the ice-ten atomic layers to give an orthorhombic Pbcm structure. At higher pressure, this may be followed by the squeezing of the H-atoms from their midpoints to give a Pbca structure and then, at over a terapascal (TPa, 107 atm), to a metallic ice, consisting of corrugated sheets of H and O atoms with the H-atoms at the octahedral midpoints between next-nearest oxygen atoms [1709]. Alternative views have been given; one is that the orthorhombic Pbcm structure is superseded by a Pmc21 phase above 930 GPa, followed by a P21 crystal structure at about 1.3 TPa, and finally, the metallic C2∕m phase above about 4.8 TPa [1818]. Another study shows that trigonal P3121 and orthorhombic Pcca phases become stable in the ranges 0.77-1.44 TPa and 1.44-1.93 TPa [2114], respectively. Such ices are not molecular and may be considered as protons and oxygen dianions with mobile electrons [1666] and are expected at the core of giant planets such as Jupiter and Saturn. A partially ionic phase consisting of alternate layers of OH− and H3O+ at low temperatures has been suggested [1810]. Several new phases may convert into one where the coordination number of oxygen increases from 4 to 5 with a significant increase of density [1818]. At pressures over about 5 TPa, it has been suggested that a phase splitting occurs with (the components of) H2O decomposing into a cubic Pa-3 H2O2 -formula phase and a hydrogen-rich phase, with metallization predicted at a higher pressure of just over 6 TPa [2114].
A new superionic phase has been proposed with an approximate triple point of about 1000 K, 40 GPa with liquid (supercritical and ionized) water, and ice-seven at high temperatures (≈ 1500 K) [1572]. In this phase, the hydrogen ions (protons) were expected to be highly mobile, behaving like a liquid and moving within the solid lattice of oxygen ions. A high ionic conductivity above 100 S ˣ cm−1, comparable to metallic conductivity, is expected.
Ice-XVIII oxygen lattice (superionic fcc)
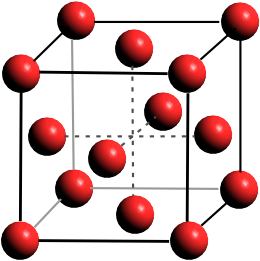
The recent experimental discovery of superionic ice has reinforced this prediction [3199]. Using shock compression of ice-seven, which simultaneously heat and compress, it was shown that this ice melts near 5000 K at 190 GPa. Superionic ice (Ice-eighteen, Ice XVIII) has been investigated using nanosecond X-ray diffraction of shock-compressed liquid water [3604]. Laser-driven shock waves can simultaneously compress and heat liquid water samples, held between the two stiff diamonds, to 100-400 GPa and 2,000-3,000 K. Under these conditions, water changes within a few nanoseconds to a face-centered cubic crystalline superionic ice (ice XVIII, see right). The edge length of a face-centered cubie is √2 ˣ oxygen diameter, and the density is 4 ˣ M/(√2 ˣ oxygen diameter)3, where M is the mass of one H2O molecule. For a density of 3 g ˣ cm−3, the oxygen diameter is 0.2416 nm, and the unit cell has an edge of 0.3417 nm. Ice XVIII appears stable at pressures higher than 100 GPa and at very hot temperatures above 2000 K. At much higher temperatures (> 4,500 K), the ice melts [3604] (see phase diagram below, noting that the surface of the Sun is about 5,800 K).
Ice-XVIII phase diagram, based on [3604]
![Ice-XVIII phase diagram, based on [3604] Ice-XVIII phase diagram, based on [3604]](images/phasexviii.gif)
Solid ice is stable to over 200 GPa and 4400 K in a body-centered cubic structure [4424], near the melt boundary in water-rich Neptune-like planets. This may be responsible for the increased electrical conductivity that may promote the generation of multipolar magnetic fields.
Due to the fluidity of the protons in Ice XVIII, and in contrast to the low-pressure ices, this ice is likely to be black. The oxygen ions form a close-packed structure with 12 nearest neighbors to each site, filling 74% of the space (cf. body-centered cubic like ice X = 68%). This ice may be considered as a new state of matter, solid and liquid, at the same time, similar to a metallic structure but with free protons rather than electrons. It has been suggested that suitable conditions for Ice XVIII lie deep inside the watery giants Uranus and Neptune and may be common throughout the Universe.
Ice-XIX oxygen lattice
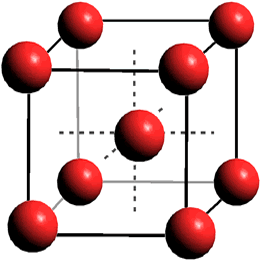
A further superionic phase of ice has been proposed [4057, 4362]. a Using combined synchrotron X-ray diffraction and optical spectroscopy of
H2O in the laser-heated diamond anvil cell up to 150 GPa and 6500 K revealed first-order transitions to an ice with body-centered cubic (bcc) oxygen atoms within a superionic hydrogen fluid above 900 K and 20 GPa. Fcc superionic ice-eighteen was found above 1300 K and 29 GPa. Its density is 1250 kg ˣ m−3 within its stability area (~30 GPa, 1250 K) [4362].
]
[Back to Top ![]() ]
]
Home | Site Index | Ices, introduction | Ice-I | Ice-Ic | Ice-Isd | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | XVI | XVII | Amorphous ice | LSBU | Top
This page was established in 2019 and last updated by Martin Chaplin on 8 February, 2022