
Clouds over the Himalayas

Cubic ice is a solid form of water that forms in high clouds.
Cubic ice (Ice Ic) is a metastable b ice crystal that can be formed (a) by condensation of water vapor at ambient pressure but at less than -80 °C, see Phase Diagram), (b) below about -38 °C in tiny droplets (≈ 6 μm diameter) [1013], and (c) by reducing the pressure on high-pressure ices at 77 K. Cubic ice is usually found as transitional states between hexagonal and cubic ice [1236c] depending on the conditions of its formation and history [1236]. Indeed, the structure of ice that crystallizes initially from supercooled water is always stacking-disordered [1236e]. Ice crystals formed on homogeneous ice nucleation in deeply supercooled water nanodrops (r ≈ 10 nm) at ∼225 K were only 78 % cubic ice with 22% hexagonal ice; effectively 44% stacking-disordered ice [3032]. It does not seem easy to prepare cubic ice crystals with a greater proportion of cubic ice structure. However, the term 'cubic ice' has been historically used for these less-than-pure ice crystals. With increasing time, metastable cubic ice converts to hexagonal ice through the increasingly disordered stacking-disordered ice.
In 2020, pure D2O cubic ice was produced from ice XVII and degassed hydrogen hydrate without any stacking defects [3857]. Such cubic ice is stable up to conversion to hexagonal ice at 250 K. Ice Ic has a (very) slightly lower density than ice Ih and ice Isd. That is why only low-density ice XVII transforms to ice Ic whereas other (higher density) ice phases yield ice Isd under similar conditions. Less complex routes to prepare 'pure' cubic ice are indicated from molecular dynamics simulations [4247].
Cubic ice structure
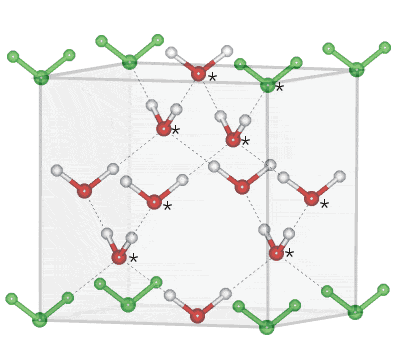
Cubic ice melts slightly lower [2559] and has a marginally higher vapor pressure than ice Ih. It may naturally form in the upper atmosphere [751], where it may form a rare 28° radius halo (usually 22° caused by hexagonal ice) around the Sun as seen by Christoph Scheiner in 1629. Cubic ice is often found in freezing confined (porous) aqueous systems. There is evidence that it may be the preferred phase for ice formed from water droplets smaller than 15 nm radius at 160-220 K [856, 996] due to its lower interfacial free energy than hexagonal ice. Larger cubic ice crystals convert, irreversibly but extremely slowly, mainly in the temperature range 170-220 K, to hexagonal ice crystals with up to 50 J mol−1 heat evolution [493]. An alternative reason for its preferable formation from supercooled water is that the hydrogen-bonded clusters present in supercooled water adopt conformations more similar to those in cubic ice [3667]. This would confirm the extensive presence of the all-gauche ten-molecule tetrahedra in the ES cluster fragments thought to be formed with supercooling.
Cubic ice consists of a face-centered cubic lattice (Space group, 227; Laue class symmetry m-3m; analogous to β-cristobalite silica) with half the tetrahedral holes filled. The starred molecules show the unit cell positions. The central cluster, (H2O)10 shown in red, exists elsewhere in a super-molecular structure [32].
ice crystals giving miller indices
(x,y,z) of faces; from [2304]
![ice crystals giving miller indices (x,y,z) of faces; from [2304] ice crystals giving miller indices (x,y,z) of faces; from [2304]](images/ice-miller.gif)
Pure crystalline cubic ice had never been found until 2020 (see above). As with ice Ih, it possesses a moderately open low-density structure where the packing efficiency is low (about 1/3) compared with simple cubic (with packing efficiency of about 1/2) or face-centered cubic (with packing efficiency of about 3/4) structures. a
In contrast to ice Ih, however, water molecules have a staggered hydrogen-bonding arrangement to all of their neighbors, rather than to 3/4 of them. In contrast to the mirrored arrangement of the layers in hexagonal ice, the cubic ice crystal possesses identical layers placed on top of one another but with displacements. The result is that the density is almost the same as ice Ih.
Comparison of the arrangements of second neighbors in cubic and hexagonal ice
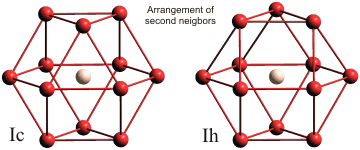
Ice Ih and ice Ic differ in the arrangement of second-neighbors (see left; the central water molecule is shown pale orange, and first-neighbor and hydrogen atoms are omitted for clarity). In ice Ic these form a cuboctahedron (far left), and in ice Ih these, form an anticuboctahedron.
Cubic ice lattice
The cubic crystal has a unit cell dimension of 6.358 Å (a, b, c; 90º, 90º, 90º, eight molecules) [383]. All molecules experience identical molecular environments. Interpenetrating ice Ic networks occur in the high-pressure ices ice-seven and ice-eight.
Cubic ice lattice, as a tetrahedron
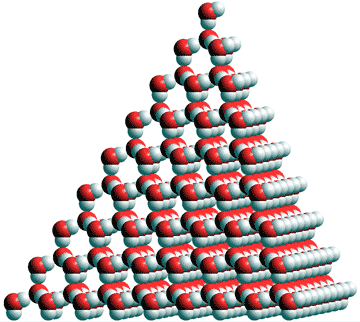
All atoms have four tetrahedrally arranged nearest neighbors and twelve second-neighbors, as ice Ih. The crystals may be thought of as sheets of chair-form hexamers in any one of the tetrahedrally arranged planes. Cubic ice contains solely chair-form hexamers in contrast to hexagonal ice that contains boat-form hexamers as well. Please note that in both these structural diagrams, the hydrogen-bonding is drawn as ordered, in contrast to the reality, it is random (obeying the 'ice rules': two hydrogen atoms near each oxygen, one hydrogen atom on each O····O bond) and protons can move between (ice) water molecules at temperatures above about 5 K [1504].
Distinct proton arrangements in cubic ice, from[2146]
![Distinct proton arrangements in cubic ice, from [2146] Distinct proton arrangements in cubic ice, from [2146]](images/cubic_ice_3.gif)
This disorder gives rise to a zero-point entropy close to 3.414 J mol−1 K−1 [2153]. c As the H-O-H angle does not vary much from that of the isolated molecule, the hydrogen bonds are not straight (although shown so in the figures). However, the ordered structure shown may exist in a proton-ordered form (ice XIc; comparable to the relationship of ice XI to Ice-Ih) [1753]. The configuration shown above is the energetically most stable form of hydrogen bonding and is ferroelectric (space group I41md, (a) left) with all H2O dipoles pointing in the same direction. A partial ordering into this configuration has been found using the methodology for producing ice XI from hexagonal ice [2146]. Three other distinct proton arrangements are the weakly ferroelectric configurations (b) (space group Pna21) and (c) (space group P41) and the antiferroelectric (d) (space group P41212), where all H2O dipoles cancel out [2146].
There are differences in the numbers of water molecules in the hydration shells around water molecules in hexagonal and cubic ices (see the Table below). Notably, an extra water molecule in the second shell of hexagonal ice helps explain its greater stability. The cavities in cubic ice are formed from ten water molecules and are smaller than those in hexagonal ice, formed from twelve. These cavities in cubic ice form a network, identical to that of the water molecules. Indeed if all the cavities contain a water molecule each, then the structure of ice-seven is formed.
Nuclear-quantum effects contribute 20 J ˣ mol−1 to the stability of hexagonal ice, making it more stable than cubic ice [3529, 3781].
Cubic and hexagonal ices have been compared [996]. Cubic ice shows an anomalous reduction in thermal conductivity with increasing pressure (as do hexagonal ice and low-density amorphous ice) but is different from most crystals. This reduction is due to hydrogen bonding changes that also decrease the transverse sound velocity [617].
Interactive structures are available (Jmol).
[Back to Top ![]() ]
]
a The exact packing efficiency for ice Ic is low
≈ 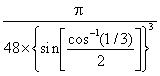 ≈ 0.34, ≈ 1/3
≈ 0.34, ≈ 1/3
compared with the simple cubic
![]() = 0.5236, ≈ 1/2
= 0.5236, ≈ 1/2
or the body-centered cubic
![]() = 0.6802, ≈ 2/3
= 0.6802, ≈ 2/3
or the face-centered cubic and hexagonal close packed
![]() = 0.7408, ≈ 3/4
= 0.7408, ≈ 3/4
[Back]
b The metastability of cubic ice relative to hexagonal ice is due to the increased symmetry of the cubic ice crystal. Although both crystal structures involve tetrahedrally placed oxygen atoms, cubic ice constricts the water H-O-H bond angle more strongly towards the tetrahedral angle (109.47°), relative to hexagonal ice, and away from its gas phase value (104.47°). The difference in energy for the pure crystals has been estimated as greater than 135 J mol−1 [2390] but may be far lower at low temperatures (<200 K) [2559]. [Back]
c Zero-point entropy is entropy (disorder) remaining even if the material could be cooled to 0 K (absolute zero, 0 K = −273.15 °C). [Back]
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Isd | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | XVI| XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2002 and last updated by Martin Chaplin on 5 August, 2021