
Ice XII substructure
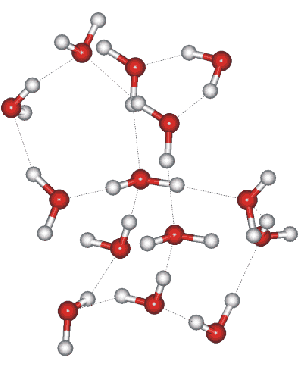
Ice-twelve (ice XII) may be formed by heating high-density amorphous ice at a constant pressure of 0.81 GPa from 77 K to ≈ 183 K at a rate of ≥ 15 K min−1 and recovered at atmospheric pressure at 77 K [386]; a slower rate (≤ 0.4 K min−1) preferably producing ice-four). Ice-twelve is metastable within the ice-five and ice-six phase space (see Phase Diagram). It forms a tetragonal crystal (Space group, 122; Laue class symmetry 4/mmm).
In the crystal, all water molecules are hydrogen-bonded to four others, two as donor and two as acceptor. Ice-twelve contains a screw-type hydrogen-bonded arrangement (right-handed double helix) quite unlike that found in other crystalline forms of ice, with the smallest ring size consisting of seven molecules ([390] not five as reported [82]; two seven-membered rings can be seen top-left and bottom right in the opposite sub-structure). It has a density of 1.30 g cm−3 at 127 K and ambient pressure, somewhat greater than ice-five (1.23 g cm−3). The hydrogen-bonding is disordered and continually changing as in hexagonal ice.
Ice XII crystal structure
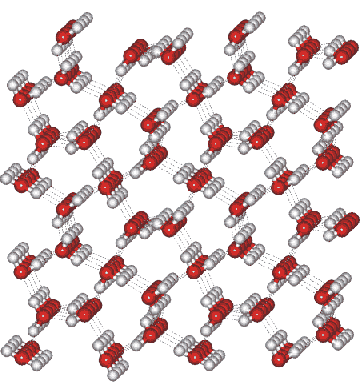
The tetragonal crystal (shown on the left) has cell dimensions a, b = 8.276 Å and c =4.027 Å (90º, 90º, 90º) and contains 12 water molecules [391]. A third of these water molecules are more regularly tetrahedral than the remainder and, thus, experience a differing molecular environment. In the above structure, the seven-membered rings involve water molecules above/below each other such as found at the bottom right.
Ice-twelve is metastable within the ice-five phase space. The near-infrared spectra of ice-twelve has been compared with that of hexagonal ice [4189].
Note that in this structural diagram, the hydrogen-bonding is ordered. In contrast, in reality it is random (obeying the 'ice rules': two hydrogen atoms near each oxygen, one hydrogen atom on each O····O bond). This disorder gives rise to a zero-point entropy close to 3.393 J mol−1 K−1 [2153].
The ordered hydrogen-bonding form of ice XII is ice XIV (ice-fourteen).
There is a reported triple point between the metastable phases of Ice XII and Ice IV and liquid water at -6 °C and ≈ 500-600 MPa [1300], but this needs confirmation.
Interactive Jmol structures are given.
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Ic | Ice-Isd | II | III | IV | V | VI | VII | VIII | IX | X | XI | XIII | XIV | XV | XVI| XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2002 and last updated by Martin Chaplin on 4 October, 2021