
Ice-thirteen (XIII)
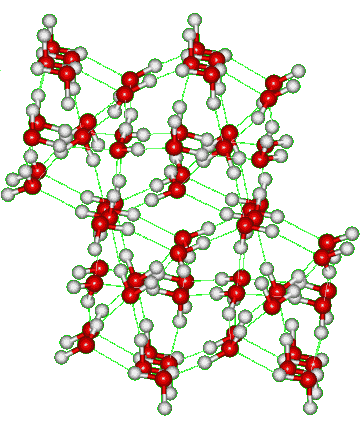
Ice-thirteen (ice XIII) is the metastable proton-ordered form of ice-five (shown on the right), formed by doping with 10 mM HCl (about one molecule to every 5000 molecules of water) below 130K and at 500 MPa to facilitate the phase transition [1002]. Other dopants have been tried [3324]. A reversible transition between ice XIII and (metastable) ice V is observed on cycling between 80 and 120 K [1058]. The crystals were monoclinic P21/a with cell dimensions a 9.24 Å, b 7.47 Å and c 10.30 Å (90°, 109.7°, 90°, 28 molecules; at atmospheric pressure and 80 K) and a unit cell contains 28 water molecules (four each of seven distinct types) [1002]. A crystallographic study of this phase transition, including the effects of HCl, HF, LiOH, and KOH doping, has been completed [4289].
The phase transition boundary between the ice phases IX and XIII have been predicted, based on the second-order
Møller–Plesset perturbation (MP2) theory, as occurring at ~0.30 GPa and 154 K [3912].
The experimental 2D IR spectra of D2O isotopically pure ice Ih, ice II, ice V, and ice XIII have been compared [3121].
Interactive Jmol structure is given.
[Back to Top ![]() ]
]
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Ic | Ice-Isd | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIV | XV | XVI| XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2006 and last updated by Martin Chaplin on 4 October, 2021