
Erasto Mpemba

“to cool hot water quickly, begin by putting it in the sun”
Aristotle, 4th century BC
"If two systems are cooled, the water that starts hotter may freeze first”
Mpemba & Osborne (1969) [1388]
![]() Hot water may
freeze faster than cold water
Hot water may
freeze faster than cold water
It is generally anticipated that cooling a hot system will take longer than cooling an identical but cooler system. Surprisingly, this is not always the case in water and magnetic alloys [3465], where it is observed that a hot system can be cooled faster. For many years, it has been known that pre-boiled water freezes before unboiled water if starting from the same (ambient) temperature.f This has been recognized even as far back as Aristotle [2043], who stated "The fact that the water has previously been warmed contributes to its freezing quickly: for so it cools sooner". Also, it is well-known that hot-water pipes are more prone to bursting in cold weather than cold-water pipes.
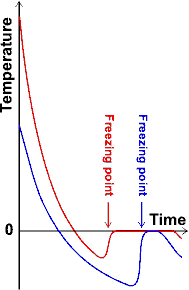
The ability of hot water to freeze faster than cold seems counter-intuitive as it would seem that hot water must first become cold water, and therefore, the time required for this will always delay its freezing relative to cold water. However, experiments show that there is a non-equilibrium process by which unstirred hot water (for example, 90 °C) does often (but by no means always) appear to freeze faster than the same amount of unstirred cold water (for example, 18 °C) under otherwise identical conditions [158] (see also graph below left [2810] ). The Mpemba effect has been proven in spin glass (a type of disordered magnet) [3708].
The Mpemba effect, 'Hot water may freeze faster than cold water', was brought to the attention of the scientific community by the perseverance of Erasto Mpemba [1388], a 13-year-old Tanzanian schoolboy a who refused to reject his own evidence or bow to disbelieving mockery, that he could freeze ice cream faster if he warmed it first. The Mpemba effect and its reverse (a cooler sample may heat faster than a hotter sample) have been shown, by theory and computation, to exist in the simple system of a uniform granular fluid [3112]. The rationale for this is that some particles may be given more energy than others, even if the mean temperature is unchanged, a theory that fits even better with the 2-state hypothesis for water. Similar behavior has been observed in carbon nano-tubes resonators [3113]
Unfortunately, the Mpemba effect (Hot water may freeze faster than cold water) is not well formulated for precise testing as the temperatures are not stated, nor is the amount of freezing. It was never meant to be precisely determinable, as are other anomalies such as the temperature of maximum density, but more as a fascinating natural phenomenon that has been noticed since the beginning of recorded history and contains an exciting and edifying story for students. No standard experimental setup is described, with various cooling regimes being used, causing differences in observations and explanations depending on, for example, the base cooling temperature. Generally, it is accepted that the typical difference in start temperatures may be about 40 °C, and the extent of freezing is that it should be visibly extensive but not complete. In the definition, the word 'may' is reasonably interpreted as 'more often than not', and certainly not as 'always'.
Proof of the Mpemba effect, from [2810]
![Proof of the Mpemba effect, redrawn from [2810], the red line is a guide for the eye Proof of the Mpemba effect, redrawn from [2810], the red line is a guide for the eye](images/Mpemba-g.gif)
Many explanations have been put forward [959, 1921].b One that has gained some support is that there is sufficient evaporation from the hot water that this causes faster cooling plus a reduction in mass, so faster cooling (even to the extent of the existence of a cross-over temperature between, initially hot and initially colder, cooling curves [1716]) and freezing [1390]. However, there does not seem to be sufficient mass lost in experiments to support such an explanation to be the sole cause and the Mpemba effect is apparent even when the vessel has a lid. Related to this explanation, part of the cause may be due to the variously sized and persistent convective currents created when hot water cools in different experimental setups [4164]. The importance of gas content has been shown to be involved with high statistical certainty [2810]. Another scenario concerns the O:H–O hydrogen bond possessing memory, whose thermal relaxation intrinsically defines the energy emission rate [2227]. This hypothesis needs further substantiation as individual bonds between neighboring oxygen atoms (of H2O molecules) have only fleeting lifetimes,d Also, there does not appear to be a good reason why the behavior of the O:H–O hydrogen bond is not fully reversible, and the described cooling curves have been challenged [2809].
Hot water freezing before cold
The initial amount of ice produced on
nucleation is about 1.3% for every
degree of supercooling.
The most likely scenario (described in [158], disputed [1415], but later supported [2047]) is that the degree of supercooling is greater, under some circumstances, in initially cold water than initially hot water. g Indeed, it has been known for over a hundred years that the thermal history of a liquid has an effect upon the kinetics of phase transformations to its solid [3330]; although often this is the opposite to that found in water with preheating increasing the supercooling effect.
In this case, the initially hot water appears to freeze at a higher temperature (less supercooling). However, less of the apparently frozen ice is solid, and a considerable amount of still-liquid water remains trapped and hidden under an outer ice layer. Initially-cold water freezes at a lower temperature to a more completely solid ice with less included liquid water; the lower temperature causing intensive nucleation and a faster crystal growth rate. If the freezing temperature is kept at about -6 °C, then the initially hot water is most likely to (apparently) freeze first. If freezing is continued, initially cold water always completely freezes before initially hot water. After coming to this conclusion, it was found that this explanation for the Mpemba effect was first experimentally determined in 1775 [1861]. Many factors, including the temperature of the cooling bath, the material in the cooling bath and the physical dimensions and characteristics of the vessels all affect both the cooling rate and whether, or not, supercooling occurs [2281] such that these factors must be clearly stated and controlled. Such factors must be taken into account when comparing different experimental procedures.
Why initially cold water supercools more is explained in terms of the gas concentration and the clustering of water (an effect on entropy [2961]). Indeed, water behaves differently and possesses a different structuring at the same temperature depending upon whether it is being heated or cooled [1697]. Icosahedral clusters do not readily allow the necessary arrangement of water molecules to enable hexagonal ice crystal initiation; such clustering is the cause of the facile supercooling of water. Water that is initially cold will have the maximum (equilibrium) concentration of such icosahedral clustering. Initially-hot water has lost much of its ordered clustering (higher entropy [2961]) and, if the cooling time is sufficiently short, this will not be fully re-attained before freezing. Experiments on the low-density water around macromolecules have shown that such clustering processes may take some time [4]. Also of relevance here is that the formation of clathrate ices, which have structures closely related to the icosahedral clusters, behaves oppositely. Thus, their supercooling (before clathrate ice formation) from hot water is far greater than that from cold water [1391]. c The basis of this explanation has also been given, subsequently based on differences in hydrogen bonding within clusters [2999].
It has been discovered that the charge on the liquid interface affects the freezing point of supercooled water [1737]. As the surfaces of nanobubbles are thought to be negative, such nanobubbles, with their extensive surface area, are expected to increase supercooling. Heating water containing nanobubbles is expected to destroy nanobubbles as they grow in size due to the lower gas solubility at higher temperatures and dissipate. The Mpemba effect is then simply explained by the loss of nanobubbles in hot water, which are kinetically too slow to reform on cooling. A theory has been presented for the role of micro/nanobubbles in accelerating heat transfer, and that may increase convective heat
transport [4240].
It is also possible that dissolved gases may encourage supercooling ([2810]; mouse over the figure above to see the effect of removing the gases) by increasing the degree of structuring by hydrophobic hydration in the previously cold water relative to the gas-reduced previously-hot water. The critical effect of low dissolved gas concentrations on water structure is reported in [294] with re-equilibration reported as taking several days. Also, an increase in the pressure as gas comes out of the solution when the water starts to freeze, lowering the freezing point and reducing the tendency to freeze (see the guestbook). Also, tiny gas bubbles (cavities produced on heating), with their more extensive liquid-gas interface, may increase the rate of nucleation, reducing supercooling [428]. Another possibility has been described depending on changes in any dissolved material with temperature (such as the reduction in bicarbonate in heated 'hard' water), but this has not yet been experimentally tested [1014]. The rationale for the Mpemba effect, in this case, concerns differences in the solute concentration at the ice-liquid interface, causing a localized lowering of the melting point [1014].
Rarely does this website take the trouble to criticize scientific publications, but a 2016 paper that appeared in Nature, Scientific Reports [2809a], together with its BBC radio commentary, are highly misleading and require a response. e The Mpemba effect concerns supercooling, and the formation of ice, whereas this paper only involves cooling to 0 °C or 4 °C, but (importantly) avoids supercooling and ice formation and so, despite its title, is not concerned experimentally with the Mpemba effect but with the simple cooling of liquid water. The paper shows that cold water cools to 0 °C faster than hot water. This seems to support the view given above that the Mpemba effect is down to differences in the supercooling of previously hot and cold liquid water. The paper [2809a] neither proves nor disproves the Mpemba effect;' Hot water may freeze faster than cold water'. e
As a postscript: a further paper by Burridge [2809b] supports his earlier paper's mistaken 'definition of the Mpemba effect'. However, it does not introduce any papers that support his invented definition. In this new and interesting paper, he concludes, "the initially warmer sample ... is observed to achieve complete freezing in a significantly shorter time than the initially cooler sample", using non-identical duplicates but dependent on the roughness of the containers, and hence the supercooling.
[![]() Anomalies page: Back to Top
Anomalies page: Back to Top ![]() ]
]
a Erasto Mpemba is now enjoying his retirement from being Principal Game Officer in Tanzania. He describes his discovery on YouTube. [Back]
b The winning entry in the Royal Society of Chemistry competition concerning the Mpemba effect is given here. [Back]
c The gas-hydrate clathrate ices also show an anomalously low dissociation rate [1539] that fits well with this hypothesis. Later work has shown ambiguous results [2817]. [Back]
d The O···H-O hydrogen bond has a lifetime of ≈ ns and the O···H-O covalent bond has a lifetime of ≈ ms. Assoc Prof Sun Changqing has vehemently disagreed with my assessment of this area of science, stating (rather obtusely) 'Fleeting times have nothing to do with the relaxation time for the energy "emission-transportation-dissipation" in the "source-path-drain" cycle system at all. ', Please see his papers [2227] for further details of his work. [Back]
e Burridge and Linden’s paper [2809] misstates what is known as the Mpemba effect [2877]. In their Abstract, the authors state that the Mpemba effect is “the assertion that it is quicker to cool water to a given temperature when the initial temperature is higher”. However, the early paper by Mpemba and Osborne [1388] states, “If two systems are cooled, the water that starts hotter may freeze first”. Follow-up papers (for example [158, 959, 1921, 2810]) always have included the process of freezing within the Mpemba effect. In the Royal Society of Chemistry’s competition inviting discussion of the effect in 2012, the Mpemba effect was defined as “The Mpemba effect is the phenomenon where hot water freezes quicker than cold water” and the competition received 22,000 entrants. A similar invitation from the Journal 'Temperature' (ISSN: 2332-8940) gave its definition as "warm water freezes more quickly than cold water" [959c]. All previous discussions of the Mpemba effect have involved a visible formation of ice and include the observation that hot water initially freezes faster than cold water, at least some of the time.
Burridge and Linden’s paper takes the view that “Broadly speaking, when two samples of water are cooled to the same temperature, in the same manner with the two samples being identical except for their initial temperature, and the initially hotter sample cools in less time, one can consider the Mpemba effect to have been observed”. This mis-definition of the Mpemba effect considers cooling to a low temperature to be (mistakenly) the same as cooling to, and including, visible ice formation. Their paper presents experimental results, but only for the cooling process to temperatures well above those of freezing water and certainly not including the freezing process, which was never observed.
Burridge and Linden dismiss all prior studies that included supercooling and freezing with a blanket statement that they are not meaningful. In so doing, they reject the observations of many scientists, including G. S. Kell [1390], without presenting their experimental evidence and with no support from any prior experiments. Of the Vynnycky and. Kimura study [158], they state “exhibited no evidence of the Mpemba effect”, but the paper states the contrary in its Abstract “the effect of supercooling leads to a spread in the experimental freezing times, giving results that constitute evidence of the occurrence of the Mpemba effect”. Burridge and Linden disregard data in this paper as it “cannot be fairly included in our analysis, since we exclude the freezing process”.
It is unsurprising that Burridge and Linden state “We conclude that despite our best efforts, we were not able to make observations of any physical effects which could reasonably be described as the Mpemba effect” as they never performed any experiments nor made any associated observations in any attempt to do so. It is also clear from their paper that Burridge and Linden misunderstand the correct and widely-understood definition of the Mpemba effect
There have been many attempts to explain the Mpemba effect, with supercooling often being cited. Thirty years ago, Wojciechowski et al. [2810] concluded that dissolved gases caused this supercooling anomaly with persuasive statistically meaningful (P < 0.001) experimental results that Burridge and Linden failed to find.
To conclude, Burridge and Linden’s paper neither proves nor disproves the Mpemba effect and has a misleading Title and Abstract.
Burridge and Linden's Reply
Burridge has replied to the points above, and his unedited reply is given below even though it is mistaken, misleading and highly contentious. It also misstates comments made in this website concerning ice formation.
'Thank you for sending these papers. I met with Professor Paul Linden, who as my co-author is well aware of all of our communications, and we discussed your opinions and the specific papers which you consider to be evidence.
We regard the results of the 2015 paper published by the group at KTH as very strong evidence that our conclusions are entirely valid. These experiments were carried out carefully, were well documented and used current technology to improve precision - they show evidence that the Mpemba effect can be observed but also strong evidence that the Mpemba effect cannot be observed in a repeatable meaningful way paper - this is precisely what we concluded.
With regards to the paper from the 1980's, I am disappointed that I had not found this paper prior to publishing our article - I would have liked to include reference to it, and comment upon it. However, Paul and I agree that it would not have changed our conclusions nor the degree of certainty with which they hold true.
The data included is interesting. However, like much of the work published on the Mpemba effect, it is fundamentally flawed. The paper does not clearly define nor describe details underpinning the matter of primary concern, i.e. how the to time for freezing to first occur was robustly determined. There are many issues concerning the formation of ice crystals at the smallest scale which are not discussed, for example, as you pointed out they can often melt back into the liquid state before ultimately freezing again. These, and numerous other experimental issues, result in observations of the time-for-freezing-to-first-occur being notoriously difficult to make in a robust and repeatable manner. Without discussion of all of the experimental difficulties, mitigating procedures, and evidence as to the extent of their precise effects, it is impossible to know if these results are statistically significant or not. As such, we still feel entirely able to stand by the conclusions of our paper.
However, if you are able to repeat this data with documented evidence of all mitigating procedures, error bounds and uncertainties then we would be delighted to review this data and once again consider whether our conclusions remain valid.
In the absence of this data, I will cease to respond to any communications, since without this data our communications have ceased to be fruitful.'
[Back]
f The Supposed Effect of Boiling upon Water, in Disposing It to Freeze More Readily, Ascertained by Experiments. By Joseph Black, M. D. Professor of Chemistry at Edinburgh, in a Letter to Sir John Pringle, Bart. P. R. S. Joseph Black, Philosophical Transactions (1683-1775), Vol. 65 (1775), pp. 124-128. [Back]
g Others show the possibility of the Mpemba effect occurring (under certain conditions) due to differences in heat removal during the establishment of thermal equilibrium [4173]. [Back]
![]() Phase anomalies (P1-P13) explanations
Phase anomalies (P1-P13) explanations
![]() Density anomalies (D1-D22) explanations
Density anomalies (D1-D22) explanations
![]() Material anomalies(M1-M16) explanations
Material anomalies(M1-M16) explanations
![]() Thermodynamic anomalies (T1-T11) explanations
Thermodynamic anomalies (T1-T11) explanations
![]() Physical anomalies (F1-F10) explanations
Physical anomalies (F1-F10) explanations
Home | Site Index | The anomalies of water | Water: Introduction | The icosahedral water clusters | LSBU | Top
This commentary was established in 2006 and put on its own page in 2014 being last updated by Martin Chaplin on 11 October, 2021