
Hydroxide ion
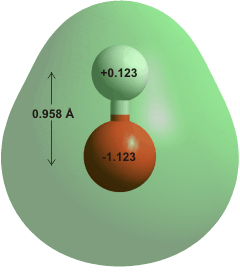

Hydroxide ions are molecular ions with the formula OH−, formed by the loss of a proton from a water molecule.
![]() pH
pH
![]() Hydrogen ions
Hydrogen ions
![]() Grotthuss mechanism
Grotthuss mechanism
The hydroxide ion is the strongest base that can exist in an aqueous solution. The structure and dynamics of the hydroxide ion in water have been reviewed [2628]. The hydration of the hydroxide ion (OH−)d is very important for both biological and non-biological processes. b Unfortunately, hydroxide hydration is neither well-known nor simply described. Most experimental structural work on this hydrated ion involves concentrated or very concentrated solutions containing structure-disruptive cations. Within such experimental environments, the basic tetrahedral structuring of water is destroyed, and many hydrogen bonds are broken. Also, the specific effects of solvent-separated and contact ion-pairs confuse any results. However it is clear that the hydroxide ion is strongly hydrated, but the extent of this hydration is less clear.
The hydroxide ion, shown above right, a strongly interacts with other water molecules to give clusters and is essentially absent (as such) in an aqueous solution. All the occupied molecular orbitals of OH− are on another page.�
Although many studies have attempted to determine the preferred hydration of the hydroxide ion in solution, there is no consensus. In particular, the hydrogen bonding capacity utilizing the donated OH− proton remains in doubt. Studies indicate that any such bond must be very weak if formed, and may be essentially absent. The vibrational spectra of aqueous hydroxide ions have been determined using two-dimensional infrared (2DIR) spectroscopy [2151].
OH− has an effective ionic radius of 0.110 nm [1946], somewhat less than that of the H2O molecular radius (0.138 nm). Its molar volume is 1.2 cm3 mol−1 due to electrostriction [1946]. c The nearest aqueous oxygen atom to the hydroxide proton appears to average about 0.25 nm, almost twice the distance as in the hydroxide ions accepting hydrogen bonds (≈ 0.14 nm), well outside the normal hydrogen-bond signature distance of 0.15-0.21 nm [698] and at a distance often considered as showing no bond [173]. The O-H stretch vibration behaves as the free hydroxyl group in small gas-phase clusters [461] and both concentrated and more dilute hydroxide solutions [1229]. However, its intensity reduces, and wavenumber increases as more water molecules hydrogen bond to its oxygen atom [2229].
In solution, the hydroxide ion must be surrounded by water with orientations governed by the local polarity and the presence of counter-ions. Clearly, water molecules (rather than cationic counter-ions) will reside relatively close to the hydroxide proton. It is not surprising that this can form the fleeting hydrogen bonds described [1509], perhaps encouraged by solvent separated counter-ions and contact ion-pairing when in concentrated solution. Such bonds are, however, far weaker than the hydrogen bonds between water molecules, as judged from their bond length and longer wavelength (i.e., blue-shifted rather than the usual red-shift generally noted for hydrogen bonds [1735]), and not readily formed [1510]. Even in ab initio calculations, no local minima is found for a water hydrogen-bonded to the OH− proton unless it is held by an extensive (and somewhat unlikely) network of bridging hydrogen bonds from 16 other water molecules [1511], The free hydroxide O-H stretch is found at higher frequency by Fourier transform infrared (FTIR) spectroscopy of HDO isotopically diluted in H2O [1512], and this is indicative of very weak or absent true hydrogen-bonding. It is probable, however, that a fleeting very weak hydrogen bond may facilitate the OH− transport mechanism [650]
The hydrated hydroxide ion (H3O2−)
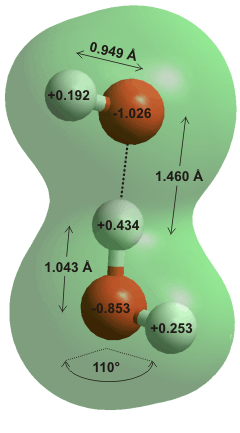
The hydroxide ion (OH−) is a very good acceptor of hydrogen bonds. The first water molecule binds strongly to form H3O2− (right, where the proton is off-center, giving rise to a low-barrier hydrogen bond) hydrated ions. a A centrally-positioned proton within the hydrogen bond (similar to that in the H5O2+ ion, 'Zundel cation') [1648] does not appear to be stable, however. Although evidence for this H3O2− ion has been challenging to find in aqueous solution [1512], it is expected to possess a particularly strong hydrogen bond [102], but infrared spectroscopy indicates that it may only last for 2-3 vibration periods (≈ 110 fs) [1647].
The hydrogen atom lies significantly asymmetrically in H3O2− where the energy barrier for proton transfer has been calculated (≈ 0.9 kJ ˣ mol−1) to be much lower than the available vibrational energy, allowing easy equilibration of the proton's position [337] as occurs with H5O2+. The vibrational spectrum of H3O2− indicates that it behaves as a single (vibrationally-averaged) species with no bend vibration (v2), both free O-H stretch vibrations being equivalent and a very strong and sharp band at 697 cm−1, corresponding to the vibration of the shared proton [755]. Thus the shared proton hops between the two minimum energy sites giving a quantum-averaged structure, similar to what may happen with H5O2+, which also shows a strong, sharp peak (at 1090 cm−1) for its shared proton. As expected, these spectra will be very much broadened, shifted, and poorly resolved in bulk liquid water.
In crystal structures, H3O2− with a symmetrical hydrogen bond may be found (for example, O···O 2.41 Å; O···H 1.205 Å; O-H 0.733 Å [380]).
All the occupied molecular orbitals of H3O2−, found using the 6-31G** basis set, are on another page.
Ab initio studies show that up to four further water molecules may hydrogen bond around the oxygen atom of the OH−, as its charge density is spread out and not tetrahedrally situated. As the hydration increases, the hydroxide O-H bond becomes shorter, its hydrogen atom more positive, and its oxygen atom less negative. The hydrogen bonds become longer and individually weaker, whereas the hydrogen-bonded water molecules become less polarized. Using atom-bond electronegativity equalization method fused into molecular mechanics (ABEEM/MM), the populations of three-, four-, and five- coordinated OH− were 29.6%, 67.1%, and 3.4% at 298 K [4095].
The O···O distance in H7O4− and H3O2− are slightly greater (≈ 2.67 Å and ≈ 2.50 (2.467 Å [337]) respectively) and the O-H slightly shorter (≈ 0.98 Å and ≈ 1.05 (1.125 Å [337]) respectively) than in H5O2+. The hydration of these ions reduces their chemical activity, which may factor in their increased reactivity when subjected to hydrogen-bond disrupting electromagnetic effects [454].
Hydrated hydroxide ion (H7O4−),
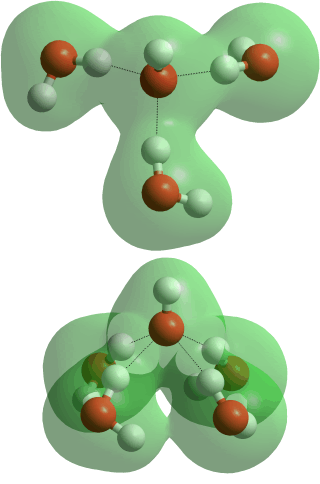
The tetrahedral ion H7O4−, see HO−··(HOH)3 above opposite, is probably the most stable hydrated hydroxide ion [541] being slightly energetically favored over H3O2− (above) [102]. It hydrogen bonds well at the surface of small clusters and even in the gas phase [1513]. It is also possible that four water molecules may coordinate to the hydroxide ion as HO−(··HOH)4, (all donating their hydrogen atoms, see below opposite) because the electron distribution around the hydroxide ion is not directional [371]. This arrangement has been reported using neutron diffraction, with empirical structure refinement, [698], and is consistent with X-ray absorption spectroscopy of concentrated solutions [1510], with both studies utilizing concentrated hydroxide solutions. It should be noted, however, that at such high concentrations, most, if not all, water molecules must be within the first shell of at least one ion [650], and the normal tetrahedral clustering of water, as found in more dilute solutions, has been destroyed. Indeed, the Raman spectra of hydroxide solutions change when the solution is diluted below OH−:H2O 1:20 [1229]. Also, HO−(··HOH)4 was found to be energetically unfavorable using quasi-chemical theory [541] and spectroscopic studies indicate the 4th H2O in HO−(··HOH)4 to be preferably hydrogen-bonded to the other three forming a second shell [461]. First shell HO−(··HOH)4 and HO−(··HOH)3 clusters cannot be distinguished using 2DIR spectroscopy [2151].
Hydration of OH− at low H2O
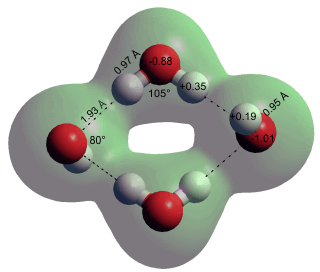
Shown left is the geometrically optimized O4H6−2 rhombus water-hydroxide ion complex formed under low hydration levels [4242].
The O5H8−2 trigonal dipyramid hydroxide pair reported in [4242] was not formed using ab initio calculations using the 6-31G** basis set
The strong hydrogen bonding between the hydroxide ion (OH−) and its first shell water molecules is thought responsible for the substantial temperature dependence of the hydroxide reorientation, with a three-fold increase in activation energy at low temperatures (<290 K) [1515]. Although thought possibly due to the presence of hyper-coordinated HO−··(HOH)4 clusters [1515], such an effect could equally well be due to dominant tetrahedral HO−··(HOH)3 clusters at low temperatures, fitting better into the more extensive tetrahedral network of water molecules then present. This reorientation effect seems to indicate a changing hydration structuring around the hydroxide ion with temperature. It is not proven that the planar HO−(··HOH)4 ion (bottom opposite) has importance in dilute solutions beyond its, perhaps transient, formation during diffusion. Also, it certainly appears that HO− ions enhance tetrahedrality in the overall hydrogen-bond network of water [2042].
At very high hydroxide concentrations, up to about two molecules of water per hydroxide ion, there is considerable ion-pairing to the cation present, ion sharing, and inter-ligand hydrogen-bonding to water [2834].
[Back to Top ![]() ]
]
a The hydroxide ion and small hydrated hydroxide clusters shown on this page were drawn using ab initio calculations using the 6-31G** basis set. Where not otherwise referenced, bond distances, angles, and atomic charges were derived from these calculations. [Back, 2]
b OH− + aq ![]() OH− (aq) (ΔG° = -437.6 kJ ˣ mol−1, assuming that the standard state of the solvent water is taken as 1.0 M). [1067]). [Back]
OH− (aq) (ΔG° = -437.6 kJ ˣ mol−1, assuming that the standard state of the solvent water is taken as 1.0 M). [1067]). [Back]
c !00 g of NaOH can be added to one liter of water without any change in the final volume of the solution (1 L, 2.5 M). [Back]
d The hydroxide ion is usually shown as OH− but this is to be taken to mean that the negative charge is associated with the molecular structure OH not just the pendant H. More accurate would be [OH]− but this is rarely used. An equivalent structural form is the seldom-used −OH, (OH− ≡ −OH). [Back]
Home | Site Index | The water molecule | Hydrogen ions | H3O+ and OH− molecular orbitals | H3O2− molecular orbitals | H5O2+ molecular orbitals | LSBU | Top
This page was established in 2008 and last updated by Martin Chaplin on 7 October, 2021