
Liquid water

Liquid water has several fundamental properties that define its unusual behavior.
'Water is the driver of Nature'
Leonardo da Vinci
A single water molecule
Liquid is a fundamental state of matter. It is much denser than the gas and has a specific volume dependent on its temperature and pressure. It does not have a fixed shape and can flow and conform to the shape of a container, unlike the solid-state. Over a limited range of temperatures and pressures, the liquid can coexist with the solid or gas phases. The density of a liquid is similar to but generally less than its solid, but this does not hold for liquid water that is denser than its commonly found solid ice. Among the three fundamental states of matter, gas, liquid, and solid, the liquid state is the most poorly understood [4384, but see 4402] far from the better-understood properties of a low-density gas or an ordered crystal. The significant difference between gases and liquids is the frequency of collisions in the liquid state, causing strong correlations between the positions of the particles. Of all liquids, water stands out for its peculiar properties and is the most extraordinary substance. Its properties are a challenge, particularly as it possesses such a simple molecular formula. On this page, some of its defining properties are described.
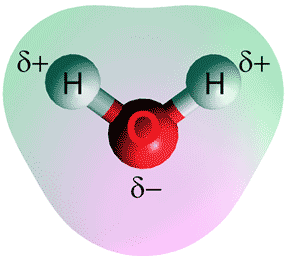
The water molecule is bent (see left), and has two hydrogen atoms and one oxygen atom. The hydrogen atoms are slightly positively charged, whereas the oxygen atom has a negative charge. A locally tetrahedral four-fold hydrogen-bonding pattern (see below left) characterizes ambient water. As the pressure is increased (towards 1 GPa) up to two further water molecules are pushed into the first coordination sphere without establishing any extra hydrogen bonds. These fifth and sixth neighbors may be found when modeling at lower pressures but in smaller amounts [3658].
The molecules can interact with each other in three ways, hydrogen-bonding, van der Waals interactions, and electrostatic interactions. Most importantly, they can form hydrogen bonds with each other. These are moderate forces that connect the oxygen atoms of neighboring water molecules by means of single hydrogen atoms donated by one of the water molecules. A water molecule can, therefore, form extensive chains (see below right).
A chain of water molecules; a water wire
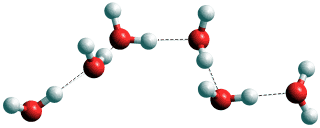
Such chains can form complex three-dimensional networks. In liquid water, the network is a 3-D percolating infinite cluster. Each linkage in such networks only lasts a short time (~ ns), such that the network is in a continual structural flux. However, the connections between water molecules within the network transmit structural information throughout the liquid. The hydrogen bond network fluctuates with both more static and released swift motions, and including density fluctuations and energy flows [4429].
Hydrogen-bonding between neighboring water molecules and the high density of molecules present due to their small size, produces a strong cohesive effect within liquid water that is responsible for water's liquid nature at ambient temperatures. In addition to this complex connected system, van der Waals interactions act locally around each water molecule to hold and orient unbound water molecules. Electrostatic effects act at all distances (if decaying with distance) around the molecules to orient the water molecules. The combination of these three interactions gives rise to a two-state structuring of high-density and low-density networks.
Mobile protons and electrons
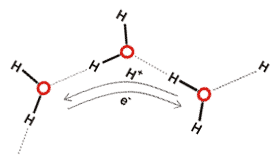
In the liquid state, the three atoms do not stay together. The hydrogen atoms constantly exchange between water molecules due to protonation/deprotonation processes and transfer along the water wires. Both acids and bases catalyze this exchange. Even when at their slowest (at pH 7), the average time for the atoms in an H2O molecule to stay together is only about a millisecond. However, as this brief period is much longer than the timescales encountered during investigations into water's hydrogen-bonding or hydration properties, water is often treated erroneously as a permanent structure.
Unlike most materials, the water dissociates,
2 H2O(aq) ![]() H3O+(aq) + OH−(aq)
H3O+(aq) + OH−(aq)
Water can support acid-base equilibria over an extensive range. As a solvent, water can both accept protons from acids and donate protons to bases, so revealing its amphoteric character, a property of great importance to life as we know it.
A typical tetrahedral group of five water molecules (the
Walrafen pentamer). In the diagram, the central H2O
donates hydrogen bonds to the top and front and accepts
hydrogen bonds from the side water molecules.
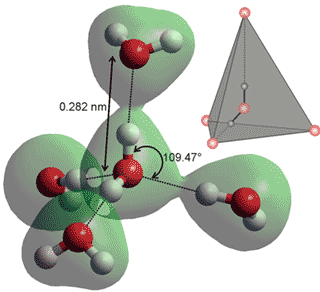
In liquid water, a all water molecules have at least one hydrogen bond to neighboring water molecules, with effectively no free water molecules to be found under ambient conditions. Water consists primarily of a mixture of clusters of water molecules, with different degrees of hydrogen-bonding, existing in rapid and complex equilibria. There is localized ordering driven by the symmetry-selective directional hydrogen bonding [3731]. Liquid water contains a mix of short, straight, strong hydrogen bonds and long, weak, bent hydrogen bonds with many intermediate forms between these extremes. This is equivalent to a simple equilibrium between extreme examples of these clusters; low-density clusters consisting of mostly tetrahedrally hydrogen-bonded water molecules (see left) and high-density clusters with many long, weak, and bent hydrogen bonds plus many adjacent, non-hydrogen bonded, van der Waals water-water interactions. The unusual variations with temperature and pressure of many physical properties of liquid water (the anomalies of water) are most easily explained using this two-state model, which is also supported by many experimental data.
Liquid water contains the densest hydrogen bonding of any material, with almost as many hydrogen bonds as there are covalent bonds. The hydrogen bonds can rapidly rearrange in response to changing conditions and environments (for example, solutes). The hydrogen-bonding patterns are apparently random in water but influence each other. For any water molecule chosen at random, there is an equal probability (50%) that the four hydrogen bonds (the two hydrogen bond donors and the two hydrogen-bond acceptors) locate at any of the four sites around the oxygen. Water molecules surrounded by four hydrogen bonds tend to clump together, forming clusters for both statistical and energetic reasons. Hydrogen-bonded chains (that is, O-H····O-H····O) are cooperative, with the breakage of the first bond being the hardest, but then the next one is weakened, and so on. Such cooperativity is a fundamental property of liquid water where hydrogen bonds are up to 250% stronger than the single hydrogen bond in the dimer, H2O····H-OH. A strong base at the end of a chain may strengthen the bonding further. The cooperative nature of the hydrogen bond means that acting as an acceptor enhances the water molecule serving as a donor.
However, there is an anticooperative aspect in so far as acting as a donor weakens the water molecule's capability to act as another donor, for example, O····H-O-H····O. Therefore, it is clear that a water molecule with two hydrogen bonds where it serves as both donor and acceptor is somewhat stabilized relative to one where it has just two donors or two acceptors. This is why it is suspected that the first two hydrogen bonds (donor and acceptor) give rise to the strongest hydrogen bonds.
Notable amongst the physical properties of water are the opposite influences of hot and cold water, with behavioral changes being more accentuated at low temperatures where the properties of supercooled water often diverge notably from those of warmer water. As cold liquid water is heated individual molecules shrink, bulk water shrinks and becomes less easy to compress, its refractive index increases, the speed of sound within it increases, gases become less soluble, it takes less energy to heat, and it conducts heat better. In contrast, as hot liquid water is heated, it expands, it becomes easier to compress, its refractive index reduces, the speed of sound within it decreases, gases become more soluble, it takes more energy to heat, and it is a poorer conductor of heat. With increasing pressure, individual water molecules expand, cold water molecules move faster, but hot water molecules move slower. Hot water freezes faster than cold water, and ice melts when compressed except at high pressures (> 1 GPa) when liquid water freezes when compressed. There is a general and broad review concerning water structure and physical properties (2022) that attempts to differentiate experimental evidence from theories. [4450].
The dielectric constant (permittivity) of liquid water is unusually high due to its high concentration of dipoles enhanced by the correlations of their dipole orientations. This endows water with excellent solvent properties for salts. Water is also an excellent solvent for hydrophilic solutes, such as low molecular weight alcohols. Hydrogen-bonding is responsible for this property as they help the solubilization of hydrophilic molecules with oxygen and nitrogen atoms. The solubilities change significantly with temperature, and these changes correlate with the hydrogen-bonding donation and acceptance changes in the water.
[Back to Top ![]() ]
]
a Compared with the structures of gases and solids, the structuring in liquids is more complex and far more challenging to understand and describe. Usually, the atoms (molecules) in a solid spend their time in fixed (equilibrium) positions,b whereas those in an ideal gas spend their time in independent motion, and the sample is completely isotropic. Even if the gas is non-ideal, the deviations from being isotropic are minimal and may be considered as perturbations. In liquids, the interaction between particles is not strong enough to hold them in fixed positions, with long-range spatial periodicity. However, neither are they so weak as to allow them total freedom of movement. [Back]
b Water ice is an exception to this, as its hydrogen atoms move around. [Back]
[Back to Top ![]() ]
]
Home | Site Index | Water molecule structure | Hydrogen-bonding | LSBU | Top
This page was established in 2018 and last updated by Martin Chaplin on 30 May, 2022