
Formation of supercooled water on cooling
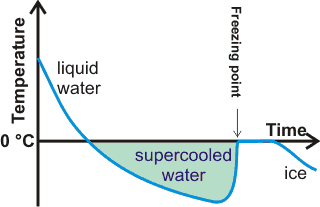
Supercooled water remains liquid below its melting point.
![]() Ultra-viscous water and the glass transition temperature
Ultra-viscous water and the glass transition temperature
supercooling of water was discovered by
D. G. Fahrenheit (1686-1736)
Volume difference (Δν) between supercooled water and solid ice.
from [3434]
![Volume difference (Δν) between supercooled water and solid ice, from [3434] Volume difference (Δν) between supercooled water and solid ice, from [3434]](images/supervol.gif)
Cooled below its melting point, liquid water is thermo-dynamically less stable than ice but typically remains liquid (in a metastable state) for a few degrees below 0 °C [1573]. It then forms solid hexagonal ice if shaken or after seemingly random periods of time (see above right). Simple molecules like water usually crystallize easily and supercool just a few degrees. Water is an exception to this generalization was indicated by Frank [4403] as likely due to geometrical frustration associated with icosahedral ordering (see also water clusters). If it is very pure and cooled very quickly or carefully without vibration, the liquid water may supercool further and occasionally to a minimum temperature of about -42 °C, or even lower temperatures at higher pressures or shorter times (≈ -46 °C (H2O), ≈ -40 °C (D2O), ≈ ms, [2706, 3134, 3837]). At about this temperature (≈ -45 °C), the density of supercooled water equals that of hexagonal ice (see left). Above this temperature, the density of ice is lower. Below this temperature, the supercooled water spontaneously crystallizes to hexagonal ice almost immediately (~ms).
Many of the properties of supercooled water (with advances described and discussed extensiely in [4342]) are anomalous, such as on heating (supercooled) cold liquid water, individual molecules shrink, bulk water shrinks and becomes less easy to compress, its refractive index increases, the speed of sound within it increases, gases become less soluble, and it is easier to heat and conducts heat better. Supercooled water has anomalously high viscosity with non-Newtonian behavior characterized by a power-law shear-thinning regime [4149].
Supercooling (as low as -20 °C) of large volumes of water (up to 100 ml) for extended periods (as long as 100 days) simultaneously can be achieved by the surface sealing of the water by an oil phase [3233]. Many factors can cause supercooled water to crystallize, including vibration, particulate initiators, and neutrons but not electromagnetic irradiation [3402]. The freezing process for supercooled water takes place in two stages. The first stage produces a distinct peak of infrared emission (heat release) with the rapid but short-lived appearance of dendritic needles inside a spongy ice-water mixture [3638]. This is followed by the slower freezing of the bulk.
Water activity of supercooled water in equilibrium with ice Ih
from [3434]
![Water activity of supercooled water inequilibrium with ice Ih, from [3434] Water activity of supercooled water inequilibrium with ice Ih, from [3434]](images/superact.gif)
A model for the thermodynamic properties of supercooled water has been developed that gives its heat capacity, vapor pressure, density, thermal expansion, and water activity (see right) [3434] (see also [3954]). As water is (super)cooled, the number of tetrahedrally hydrogen-bonded water clusters reduces, but they are larger in size [1850]. Supercooled water occurs naturally in high-altitude clouds. It possesses many properties that differ anomalously from warm water (see water anomalies) [2580]. It has been proposed that this difference in properties is due to the large proportion of the expanded ES-related clusters in the two-state mixture model of water. In brief, supercooled water contains more strong tetrahedral hydrogen-bonded water molecules [2501] and pentameric water clusters [2439], and these structures increase as the temperature is lowered. Such structuring does not readily form crystalline hexagonal ice. There is also a significant positive deviation from the extrapolated surface tension behavior below 235 K consistent with the tail of an exponential growth in surface tension as temperature decreases [2737]. This is thought to support the coexistence of two liquid forms in pure water of macroscopic size at these low temperatures. Interestingly, molecular dynamics simulations indicate that homochiral domains appear as significant constituents in supercooled liquid water [2738].
Thermodynamics of Ar-like solute hydration
from [4322]
![Thermodynamics of Ar-like solute hydration, from [4322] Thermodynamics of Ar-like solute hydration, from [4322]](images/hydrocool.gif)
The excess chemical potential (μ), enthalpy (H, and entropy (TS) of argon in supercooled and ordinary water is shown left [4432]. There are minima in both the enthalpy and entropy of hydrophobic hydration. This is in contrast to the expected monotonic temperature dependence traditionally ascribed in other liquids. The rationale for this behavior is the presence of the metastable liquid−liquid (second) critical point [4486].
There are earlier [569] and more recent [1794, 1860] comprehensive reviews of the properties of supercooled water. A two-structure approach to the description of the thermodynamic properties of supercooled and stretched water, below 300 K and up to 400 MPa and down to −140 MPa has been described. This work also clarified the concept of the fast interconversion of alternative states in supercooled water as a phenomenological representation of a distribution of short-ranged local structures. It concluded that they "hope that the two-state approach to supercooled water is more than a grossly simplified phenomenology" [3660].
As the temperature of supercooled water is lowered, local structures characterized by both LDA and HDA amorphous phases are found, with a rapid increase in the LDA-like population and linking to water’s anomalies [4153]. Using molecular dynamics simulations on supercooled water, it has been found that structural and dynamical instabilities are hidden at about 190 K in the experimentally inaccessible region between 235 K and 150 K [4145]. Here, there is a break-up of locally distorted tetrahedral structures. This behavior is what would be expected from the ES scenario on cooling, as symmetrical fully tetrahedral clusters reach the size limit of stability.
A cluster of five water molecules
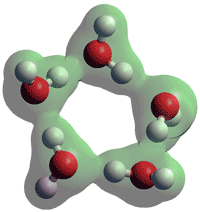
Deeply supercooled water has large density fluctuations. Modeling has indicated that these may be partially due to an increase in large voids [4052]. It has been proposed that if water could be supercooled to lower temperatures without crystallization, it may reach a liquid-liquid transition between low-density and high-density water [3420]. Such a reversible liquid-liquid transition has been found in a 0.02 molar fraction solution of trehalose at about 0.2 GPa and below 159 K [4418]. The effect of the nearby second critical point (see the diagram) causes the divergent behavior of many of pure water's thermodynamic properties [871]. Although this phase change has many supporters, it is impossible to investigate as the water crystallizes before it can reach No man's land 150 K< T < 227 K). However, a concentrated solution of the ionic liquid salt hydrazinium trifluoroacetate (N2H5 +.TFA−, xw = 0.84) allowed this phase change to be reversibly crossed [2602], although at a lower temperature (≈ 188 K) than that predicted (≈ 225 K) for pure water. Remarkably, the molecular ratio of the water to salt (≈ 5.94) that is required is very similar to that found in the clathrate hydrates (Clathrate I, CS-I 5.75; Clathrate II, CS-II 5.67; Clathrate H, HS-III 5.67; Clathrate HS-IQ 5.71). This indicates that the ionic liquid salt groups are separated by only a single water molecule layer and that the structure of LDA may include many clathrate-like structures (as this liquid ionic salt solution is proposed to be thermodynamically very similar to supercooled water and LDA [2602]). Research has further explored the edges of No man's land, giving improved estimates of the possible location of water’s second critical point [3337]. Wide-angle x-ray scattering of supercooled water down to 234.8 K shows pentamer rings in low-density liquid-like structures [3625]. Using simulations, the size of correlated molecular fluctuations increases on (super)cooling, reaching a value corresponding to a few thousand molecules, indicating the growth of spatially localized dynamic fluctuations [3782].
Supercooled water may be formed by the fast evaporative cooling of micrometer-sized water droplets in a vacuum, where a fraction of the droplets remain liquid down to 230.6 K [3298]. Supercooled water (135 K - 245 K) at the surface of Pt (111) has been examined by infra-red spectroscopy and shown to consist of a mixture of low-density and high-density clusters [4076]. This work has been extended to examine supercooled water from 170 K - 260 K [4283]. The structural relaxation was shown to always be fast compared to crystallization within the zone known as 'No man's land'. Also, the complex relaxation kinetics of its two-state mixture (in this case, low-density amorphous solid water (LDA) and hyperquenched glassy water (HQW)) are governed by a distribution of energetic barriers to rearrangements, with HQW typically relaxing more quickly than LDA no doubt due to its lower content of strong hydrogen bonds.
[Back to Top ![]() ]
]
Home | Site Index | Phase Diagram | Liquid water | Ices, introduction | LSBU | Top
This page was established in 2004 and transferred in 2016. It was last updated by Martin Chaplin on 25 September, 2022